Recommendations for adaptation and validation of commercial kits for biomarker quantification in drug development
Abstract
Increasingly, commercial immunoassay kits are used to support drug discovery and development. Longitudinally consistent kit performance is crucial, but the degree to which kits and reagents are characterized by manufacturers is not standardized, nor are the approaches by users to adapt them and evaluate their performance through validation prior to use. These factors can negatively impact data quality. This paper offers a systematic approach to assessment, method adaptation and validation of commercial immunoassay kits for quantification of biomarkers in drug development, expanding upon previous publications and guidance. These recommendations aim to standardize and harmonize user practices, contributing to reliable biomarker data from commercial immunoassays, thus, enabling properly informed decisions during drug development.
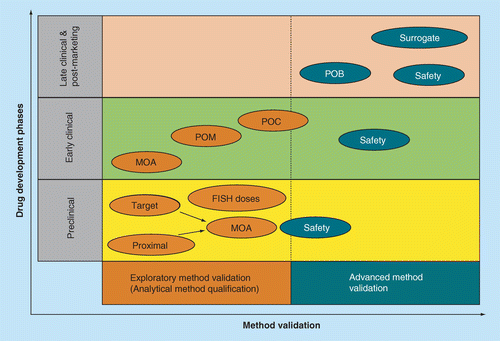
FIH: First time in human; MOA: Mechanism of action; POB: Proof of biology; POC: Proof of concept; POM: Proof of mechanism.

Today the pharmaceutical and biotechnology industries are confronted with a myriad of challenges, including increasing drug development costs, decreasing drug approvals, as well as societal pressure to reduce healthcare costs that comes with healthcare reform legislation [1]. In 2004, the US FDA published a document recognizing these issues titled “Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products” and launched the Critical Path Initiative (CPI) [2]. The goal of the CPI was “to stimulate and facilitate a national effort to modernize the scientific process through which a potential human drug, biological product or medical device is transformed from a discovery or ‘proof of concept’ into a ‘medical product’.” It was recognized that there is a need for better measures of the efficacy and safety of drugs. In this regard, an opportunity for the use of biomarkers in drug development was highlighted and has evolved into standard practice within most pharmaceutical and biotechnology companies. According to the CPI, “Additional biomarkers (quantitative measures of biological effects that provide informative links between mechanism of action and clinical effectiveness) and additional surrogate markers (quantitative measures that can predict effectiveness) are needed to guide product development.” Furthermore, in 2006, the FDA published their Critical Path Opportunities List and again identified biomarker development as one of two areas with greatest potential impact [3].
The recognition of these pharmacoeconomic realities and the potential benefit of biomarkers to improve safety, efficacy, efficiency and decision making for personalized medicine has reached widespread acceptance in the pharmaceutical industry [4,5]. It is now quite common for biomarker data to play a key role in early decision making during drug development, prior to investment in more expensive late-stage activities. Clearly, these decisions are highly dependent upon the quality of the data provided; this can only be ensured when biomarker assays are appropriately characterized and demonstrated to be suitable for their intended use. To address this need, the 'fit-for-purpose' biomarker method validation publication of Lee et al. [6] proposed a general approach for development and validation of Ligand-binding-based biomarker assays, in which the rigor of analytical validation should depend on the intended use of data. The publication focused on assays developed de novo for specific and particularly novel uses. These assays typically require in-house technical expertise and can consume extensive resources and time for adequate reagent generation, characterization, assay development and optimization. Given the desire to support biomarker hypothesis testing, which typically requires quantification of multiple analytes but with finite resources and time, pharmaceutical companies are searching for efficient alternatives to de novo developed assays. One convenient and increasingly common approach is to use commercially available biomarker assay kits to support drug development programs. Such kits are appealing since they potentially offer an expedited analytical solution, ease of operation, portability, cost–effectiveness and are less resource intensive [7,8].
Despite the multitude of readily available commercial kits from numerous vendors, the extent of method development and critical reagent performance characterization can vary significantly and can in many cases be insufficient to support the use of these assays as drug development tools for conducting analyses of samples from clinical trials [7,9,10]. As commercial kits tend to be designed and marketed for broad applications across different species and types of matrices rather than for specific applications in clinical trials, this paper offers kit users recommendations on how to evaluate, select, adapt and validate commercially available biomarker immunoassay kits in support of drug development programs. The availability of a systematic process for evaluation and application of commercial kits should help analysts with generating higher quality data and enabling better decision making.
For this paper we have endeavored to incorporate current industry best practices [6,7,11], expand upon the recommendations in the draft 2013 FDA Bioanalytical Method Validation Draft Guidance [12], as well as provide a framework that can be used for standardizing the selection and validation of commercial kit-based assays for definitive and relative quantitative determination of biomarkers in support of drug development.
Brief overview of categories of commercial kits
A brief overview of categories of commercial kits is provided to put things into context. A number of different categories of commercial kits, labels and certifications are available; all can be used in drug development for making quantitative measurements of biomarkers. These include: In-vitro diagnostic (IVD) assays [13,14], Conformité Européenne (CE)-marked assays [15], research use only (RUO) assays [14] and vendor 'qualified' assays. An understanding of these categories and their inherent differences is important for the user to understand whether a given method may be suitable for the intended use, perform additional assay adaptations(s) and characterization to meet the intended application for biomarker quantification.
IVD assays
IVD assays are products that have been cleared or approved by the FDA (defined in 21 CFR 809.33) for an intended use in clinical diagnosis or patient care (Table 1). There are multiple categories of these assays (class I, II and III) and several approval processes that include Premarketing Approval and 510(k), which are well described by FDA regulations (21 CFR 210(h), and 21 CFR Section 522). These assays must conform to strict quality system regulation for IVD devices as specified in 21 CFR Part 820 [13,14,16,17]. For example, the manufacturer must demonstrate control of inter-lot variability (see section minimization of interlot variability). Thus, these assays are well characterized and fully validated for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat or prevent disease or related sequelae [13]. However, these assays are designed and designated for a specific use. For instance, most IVD assays are approved for use with specific instruments that typically have closed systems for dedicated sample types (e.g., human plasma, serum, urine). They are also intended to perform over defined quantitative ranges of the analyte for diagnostic purposes. In some cases, treatment with drug may result in a decrease in the biomarker concentration to levels below the quantitative range for which the diagnostic was approved. Thus, IVD assays may not provide the full range and sensitivity required to support clinical biomarker measurement in different matrices and disease states.
Assays run in a Clinical Laboratory Improvement Amendments environment
Clinical Laboratory Improvement Amendments (CLIA) were established by the US Congress in 1988 to define quality standards for all non-research laboratory testing performed on human specimens for the purpose of providing information for the diagnosis, prevention, treatment of disease or impairment of or assessment of health [18]. Laboratories in the USA that perform clinical diagnostic tests must meet CLIA requirements, which are primarily enforced by the Centers for Medicare & Medicaid Services. The goal of CLIA is to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test is performed. CLIA certification involves having a laboratory director, supervisors and staff that all have predefined qualifications: acceptable laboratory practices for equipment and processes, acceptable performance in relevant proficiency testing programs [18].
Test methods that are performed in CLIA laboratories fall into one of three different categories (depending on the complexity): waived, moderate and high [16]. The FDA is responsible for categorizing commercially marketed IVD tests under CLIA as defined in 42 CFR 493.17. This categorization affects the stringency of the requirements under CLIA for the clinical lab or other testing sites. Categorization is based upon the following criteria:
• Technical knowledge required to perform the test
• Level of training to perform the test
• Stability and reliability of reagents and materials
• Characteristics of operational steps
• Stability of quality control and proficiency testing materials
• Test system troubleshooting and equipment maintenance
• Interpretation and judgment required to perform the test
Therefore, a test run in a CLIA-certified laboratory does not necessarily guarantee that the test has the performance characteristics desired by the end user for application as a biomarker tool in a drug development setting. The end user needs to evaluate these test attributes (such as accuracy of quantification of the biomarker in a different matrix or disease state) before deciding if these tests are suitable for their intended use.
CE-marked assays
Conformité Européenne, or “European Community” is a mandatory conformity marking for products placed in the European Economic Area (EEA). The use of the mark signifies that the manufacturer has declared and takes responsibility that the product conforms to the essential requirements of the European Community legislation, typically from a safety manufacturing perspective [15]. Thus, the CE marking on a product is a self-certification process. This enables the manufacturer to sell the product throughout the EEA. The CE mark is intended for use by the national EEA market surveillance and enforcement authorities, often for the protection of the consumer. However, for certain categories of products there are more stringent requirements needing third party review in order to obtain a CE mark. Under the IVD category this would allow an assay to be used as an IVD for a specific medical use in Europe and other countries that recognize the CE mark for marketing purposes. However, CE marked labeling does not imply or guarantee that the biomarker immunoassay will be suitable for measurement of clinical biomarkers in drug development programs.
RUO assays
Assays that are labeled as 'RUO' are exempt from regulatory requirements and approvals that would be needed for clinical diagnosis or patient management [17,19]. Thus, RUO labeling of kits is meant to serve as a warning that products so labeled should not be used in clinical diagnosis or patient management. They are intended for use only in research and discovery work. Since there are no requirements or guidelines for the RUO label, the extent of manufacturing quality compliance, assay characterization and documentation vary considerably across different vendors and assays. On the other hand, RUO kits are typically first-to-market for newly identified biomarkers and are not instrument specific. Thus, they represent an ever growing and prolific segment of the commercial immunoassay market as well as attractive tools that can be adapted for use in drug development projects (Table 1). However, adaptation of RUO kits can pose substantial challenges for conducting exploratory or regulatory-compliant bioanalysis of biomarkers in support of both preclinical and clinical drug development [7,10,19]. The common attributes of RUO kits along with the recommended adaptations for users in drug development programs are summarized in Table 2.
Vendor qualified or validated RUO assays
There are commercial kit vendors that produce RUO assays, self-classified as 'qualified' or 'validated'. The use and definition of the term is vendor-specific and may signify a higher level of manufacturing rigor, higher degree of characterization (reagents and kit performance) and some level of 'fit-for-purpose' validation. As with the RUO label designation, the use of the terms 'qualified' or 'validated' does not require approval or oversight from regulatory agencies and provides no guarantee of suitability for drug development purposes. Users can search the kit manufacturer's web sites to see if they provide such assays and are encouraged to contact manufacturers to understand the specific nature of such claims.
Recommendations for method development & validation of commercial kit-based assays
Prior to embarking upon a biomarker exploration program it can be beneficial to develop a 'Biomarker Work Plan' and define the intended use of the data that would be generated. The intended use dictates the rigor, the type of method validation, whether exploratory (sometimes referred to as analytical method qualification) or advanced method validation [6,7]. A Biomarker Work Plan is typically developed by key stakeholders and is usually company-specific. This should be followed by selection of a suitable commercial immunoassay kit for the intended analyte and performance of any necessary experiment and/or modification to render the kit fit for the intended use. The next step could be to write a method validation plan, characterize and validate the assay with a predefined level of rigor. These steps are discussed in some detail in the following sections.
Biomarker Work Plan
A successful biomarker effort should begin with a clear understanding of the specific needs of the project and intended use of the data [6,20]. A Biomarker Work Plan is recommended, wherein the aforementioned information is documented, agreed upon by key stakeholders and used to inform and justify the level of method validation required [6,20]. The plan should define the intended use of the data and should consider issues such as analyte(s) of interest, endogenous analyte stability, sample matrix, expected sample numbers over time (and thus projected numbers of kits), sample volume, the sample collection process, storage stability, analytical range, sensitivity and precision requirements. The latter depends on some understanding of the biomarker levels in drug-naive populations of interest, the effect of drug treatment and the effect size anticipated [6]. If possible, a priori acceptance criteria that would quantify the anticipated effect (change) should be included in the Biomarker Work Plan. In addition, the changes needed for adaptation of the kit-based method for the intended use and the assay parameters to be characterized or validated should be listed. It is important to note that the method validation plan remains the key document describing the analytical validation experiments and acceptance criteria; it would be a part of the broader Biomarker Work Plan. Typical components of the Biomarker Work Plan are described in more detail below.
Sample collection and range finding
Pre-analytical sample collection, processing and storage can be very important variables for bioanalysis and attainment of reliable biomarker information [21–23]. For example, a marked difference was observed for TRACP 5b concentrations in serum collected via syringe versus serum collected in bags, an observation that would not be anticipated or investigated by the kit vendor [24]. Often, for a novel biomarker, an objective is to measure differences in blood levels from healthy versus disease populations, as well as its prevalence. Thus, an initial task is to procure matrix from multiple individuals from these populations for analyte range finding. The number of individuals depends on whether information is already available from other sources and the importance of the drug development program to the company. For the range-finding studies, typically three or more individuals per group can be used for confirmation or a minimum of 10 individuals per population where information is lacking.
Define the rigor of method validation
The broad application of biomarkers throughout the drug development continuum from discovery through commercialization and post-marketing in relation to method validation is depicted in Figure 1. The fit-for-purpose paradigm [6] for bioanalytical method validation is roughly separated into two categories: exploratory method validation (also called analytical method qualification) and advanced method validation. It is the responsibility of the user and key stakeholders to determine and agree upon the level of method validation required for the intended use of the biomarker data [7].
Exploratory method validation
In the exploratory category, the scope and depth of analytical efforts should be driven by the likely impact of the data on internal decision making (Figure 1). In the preclinical phases of drug development, biomarker data are used to gain understanding of the mechanism of action of the drug or biochemical pathway of interest. Later, in the early clinical development phases biomarker data are used for proof of mechanism and proof of concept, as well as for dose selection in Phase I and in dose-range finding analyses in Phase II. Typically, exploratory method validation may be sufficient prior to quantification of biomarkers that demonstrate mechanism of action, proof of mechanism or proof of concept and some exploratory safety biomarker discovery work [25–27].
Analytical method qualification
In the biomarker field the term 'clinical qualification' is typically used to define the evidentiary and statistical process linking biological, pathological and clinical end points to the drug effect or linking a biomarker to biological and clinical end points. By contrast, the process of assessing the performance characteristics of a given analytical method is referred to as fit-for-purpose analytical method 'validation' [6]. The term 'method qualification' has previously been used within the drug development realm to describe the characterization of the performance of an analytical method. This term may have been adopted from the GMP arena. Historically, in the drug manufacturing sphere, processes and procedures are 'validated' using 'qualified' instruments. However, during the early stages of biopharmaceutical development (e.g., product characterization and comparability studies) people started using test methods that may be simply 'qualified' or characterized for their intended use [28]. Similarly, the term 'method qualification' (more appropriately 'analytical method qualification') is being used to refer to a streamlined form of method validation for biomarker bioanalytical methods used to support (mostly) non-GLP studies [29]. For all practical purposes 'analytical method qualification' is essentially similar to the 'exploratory method validation' described by Lee et al. [6].
Advanced method validation
Safety and diagnostic biomarkers may play a critical role in all phases of drug development. Quantification of these biomarkers may call for a higher level of confidence in the analytical method performance and, hence, may require advanced method validation [6] combined with exploratory biomarker validation prior to use (Figure 1) [30,31]. Moreover, most biomarker data generated during late phases are from pivotal trials. These data, such as assessments of safety and proof of efficacy, are intended to support drug applications, contributing to drug label and dosing information. Data intended for these applications should be suitably reliable and the methods should be fully validated as described in Figure 1. It should be noted that the activities of biomarker analytical method validation are quite similar to those of biopharmaceutical PK method validation [6,12].
The recently released Draft 2013 FDA Guidance on Bioanalytical Method Validation [12] has started to address the use of diagnostic kits as well as RUO kits to determine analyte concentrations in PK or pharmacodynamic studies and provided recommendations, although it is important to note that this draft is expected to be revised. In brief: standards and quality control (QC) samples should be prepared in the biological matrix of study samples (use of a different matrix should be justified); the method must exhibit sufficient precision and accuracy; specificity and analyte stability under actual conditions of use must be demonstrated; sufficient numbers of calibrator standards and QC samples of known concentrations should be used; immunological identity of kit standards and endogenous analyte (parallelism) should be evaluated; lot-to-lot-variability and comparability for critical reagents should be addressed [32,33]. Moreover, it is recommended that site-specific validation of the method be performed and any modifications of the kit assay processing instructions should be thoroughly validated and documented. A fit-for-purpose approach should be used when determining the appropriate extent of method validation of the aforementioned parameters [12].
In the following sections, we expand upon draft FDA guidance [12] to provide a framework that can be used for harmonization and standardization of kit-based immunoassay analytical validation procedures across the industry.
Selection of kit & kit vendor
Once the intended use of the biomarker data has been established and the availability of kits has been ascertained, the user must decide which ones to evaluate. When there are multiple kit sources, one should choose a vendor that provides the greatest level of assay characterization information, user-friendly technical service organization and flexibility of supplies (such as additional bulk materials). A poorly or improperly characterized kit may measure a completely unrelated analyte than that for which it is marketed, thus putting the entire, evolving biomarker program at risk [34,35]. It is the user's responsibility to identify a reliable vendor, obtain relevant data and information related to the kit and to assess the amount of work that their laboratory needs to do to fill any critical information gaps. Information from publications and experiences from colleagues may help in evaluating the potential reliability of a kit and the kit vendor [35].
Kit evaluation requires a substantial investment of time and effort; therefore, the authors advocate sharing experiences through open communication in scientific community forums such as those in the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium, the Predictive Safety Testing Consortium (PSTC) [36–38] and the AAPS Ligand Binding Assay Bioanalytical Focus Group. Although it is understood that sharing of such information needs to be guided by the constraints of intellectual property rights, if more can be done in a non-competitive space, repetitive tasks in individual laboratories could be reduced.
Feasibility assessment of commercial kits
After reviewing the availability of kits in the marketplace, a feasibility assessment and kit comparison may be needed to select the most suitable product and manufacturer that satisfy the user's requirements. A recommended kit selection process is outlined in the following section.
Kit selection process
Kits should be screened for suitability by the user as outlined by the scheme shown in Figure 2. Details of this process are suggested by the following tests:
• Calibration range verification and algorithm selection: This test consists of one or more accuracy and precision experiments using three to five levels of spiked controls (in kit calibrator diluent or matrix) and fitting the calibration curve with an appropriate algorithm. This test should give a reasonable indication of assay sensitivity and range of quantification;
• Assay selectivity/matrix interference test: In this test three to five biological samples are spiked with known amounts of a suitable biomarker standard material (e.g., recombinant protein) then recovery of the added material is evaluated;
• Dilutional linearity and parallelism test: A typical experiment consists of assaying samples at various dilutions using a minimum of three test samples containing endogenous biomarker and one or more spiked diluent samples. Parallelism demonstrates the immunological similarity between calibrators and endogenous analyte and the validity of using a surrogate matrix for calibrators. Dilutional linearity of spiked samples in the defined matrix evaluates the potential for matrix effects and prozone. If samples will need to be diluted into the range of the assay then this should be performed above the expected maximum concentration of analyte ;
• Kit specificity confirmation: Method specificity for the analyte of interest (versus structurally related analytes) can be evaluated if necessary using MS or other orthogonal method [35]. Alternatively, one can cross-check assay performance against a kit from another vendor or can obtain calibrator material from a reliable source and evaluate the dose–response relationship (calibration curve) using the kit reagents. Although it is a considerable amount of work, this step may turn out to be highly cost- and time-effective if specificity cannot be demonstrated and an alternative method must be identified and characterized [20].
Acceptance criteria for ligand-binding assays as previously published [6,39,40] and specified in the Draft 2013 FDA Guidance [12] for ligand-binding assays can be adopted to evaluate the test results. The results obtained in tests 1–3 can be included as part of analytical method validation activities and report, if it is pre-specified that the results of these tests will be included as part of the validation. This, or similar processes, can be readily and effectively used for selection of a potentially reliable commercial kit for biomarker quantification before proceeding with method adaptation and validation.
Method adaptation
'Method adaptation' entails finding solutions to attributes missing from the standard format of the selected kit (Table 2). Typical activities include the following:
• Creating additional calibrators: Typically, kits come with four or more levels or lyophilized calibrator stock. The draft 2013 FDA guidance [12] recommends the inclusion of additional calibrators to generate at least six non-zero calibrators. In order to create a calibration curve with six to seven calibrators in the dynamic range of quantification, the analyst can use the highest calibrator (or calibrator material stock solution) to create additional calibrators within the targeted concentration range by dilution with a suitable diluent, usually the stabilized buffer matrix supplied with the kit. In most cases the biomarker measurements in study samples can be 'relative' rather than 'absolute', therefore the use of a proprietary calibrator matrix should provide reliable data, as long as the matrix remains unchanged during the course of the entire analysis of the biomarker study. For advanced method validation, it may be desirable to prepare standards in the same matrix as the subject samples (unless justified otherwise, e.g., rare matrix, high levels of endogenous analyte) as stipulated in draft 2013 FDA guidance document [12]. However, from a practical perspective calibrators in biological matrix are not a viable option when using commercial kit based assays;
• Selecting an appropriate curve-fitting algorithm: Generally, the fitting of a curve is suggested by the kit vendor without specific recommendations for the curve-fitting model or weighting parameters. The analyst must determine an appropriate curve-fitting model (with constant or variable weighting factors) that would give acceptable assay sensitivity and precision. Additional calibrators (anchor points) outside the range of quantification may be required. Recommendations for judging the acceptability (goodness-of-fit) of a calibration curve are described in a publication by Findlay and Dillard [41];
• Preparation of controls in surrogate or biological matrix: Validation or QC samples at three to five levels can be prepared by spiking the reference material from the kit into the same matrix as the calibrators. Alternatively, separately purchased bulk reference material can be used to generate such control samples. However, it is the authors’ experience that separately purchased bulk reference material may not have the same performance characteristics as the calibration standard material provided in the kit. QC samples prepared in kit calibrator buffer are primarily used to attest run-to-run acceptance of the calibration curve. For 'advanced' method validation QC samples in biological matrix should be used where feasible;
• Identifying biological matrix with at least two levels of endogenous biomarkers: Given that calibrators and controls are typically recombinant proteins in proprietary matrices, it is prudent to include biological controls with endogenous biomarkers at two or more levels when available;
• Substituting the reagents: Reagents such as substrate solution, stop solution and wash buffer can be substituted with reagents commonly used in the lab. However, the analyst should evaluate the suitability of these reagents through assay performance before a change is made. For instance, where alkaline phosphatase (or horseradish peroxidase) is used as a signal-generating molecule the kit wash buffer can be substituted with a Tris-buffer (or phosphate buffer)-based washing solution;
• Bulk quantities of critical reagents: If automation will be used for the method, bulk quantities of critical reagents, such as antibody conjugates and high concentration reference calibrator material, should be obtained. The automated process must be included in the test procedure and validated. Since manufacturers can change reagent lots frequently, depending on demand, bulk reagents may be beneficial, controlling variability when supporting a longitudinal study where samples from a given subject are tested in more than one assay over the course of the trial.
Analytical method validation
The chosen kit and appropriately modified test method must be validated according to its intended use as previously described [39,40,42] and stipulated in the draft 2013 FDA guidance document [12]. The fit-for-purpose approach can be used to define the appropriate level of rigor [7,29]. The authors suggest that the following assay parameters be evaluated: LLOQ and ULOQ, selectivity, relative accuracy and precision, dilutional linearity, parallelism and analyte recovery [6,43]. Tests of lot-to-lot variability should also be performed at the earliest opportunity [32–33,43,44]. Similarly, the stability of endogenous analyte cannot be assessed until fundamental assay characteristics have been determined, but can be a critical investigation to initiate, depending on the analyte and intended disease population.
An overview and recommendations for analytical method qualification and advanced method validation are listed in Table 3 and further elaborated on in section “Commercial kit specific issues”.
Commercial kit-specific issues
Minimization of interlot variability
Longitudinal consistency of performance is essential for biomarker applications where small changes in analyte concentration over time are linked to treatment response. Procurement of a large supply of kits (or critical reagents, such as calibrator material and antibody conjugates) from a single lot, sufficient for analysis of all samples from a given study, is highly recommended [45]. It is routine for a manufacturer to change lots of critical reagents as part of the normal production cycle. Whilst these individual kit components may meet their lot-release specifications, combined together in the biological test system they may collectively contribute to altered assay performance. However, for long-term studies the use of multiple kit lots may be unavoidable. Thus, the kit must be capable of detecting a change from baseline when baseline and end point measurements are made using different lots. Although the kit vendor has a major role to play in controlling manufacturing processes in a way that limits variation between batches, the kit user should implement a defined process to monitor consistency of performance across lot changes. One simple approach is to use matrix controls (MCs) containing at least two levels (e.g., high and low) of endogenous analyte [46]. Trend analysis (e.g., Shewhart or Levey–Jennings control plots) of analyte concentrations measured in MC samples can be used to identify any noticeable performance variability over time [47]. When a significant difference in performance between lots is observed, the bias can be investigated using clinical sample batches run in parallel on 'old' and 'new' kit lots. Lot-dependent performance changes may call for use of an empirical correction factor [48], to compensate for differences in protein content and proportional errors if experimental data have been generated and are available to support this practice [45]. Significant performance variability between kit lots that remains unchecked may have a profound effect on pharmacodynamic data and their clinical interpretation.
Managing the expiration dates of kits
Documentation of reagent/kit lots, time of testing and trends in performance can be useful for monitoring kit stability over the course of longer studies. It is essential if operating under GxP, CLIA or other quality system, that the kits and any additional critical reagents be used within the expiration dates assigned by the manufacturer under specified storage conditions. However, reagents rarely become useless just after the expiration date. Defining a procedure for extension of kit expiration dates based on acceptable performance of the assay may be beneficial, especially when a large number of kits are acquired to support a study. King et al. [45] have recommended that reagent stability testing may include back-calculated concentrations of three to five QC levels when evaluating assay performance for extension of the expiration dates. Trend-analysis data (if available) should also be considered in this regard; a consistently declining trend in assay performance may prohibit assignment of longer expiration dates.
Matrix for calibrators
Kit calibrators are often provided as lyophilized material to be reconstituted in a proprietary substituted matrix, typically a protein-fortified buffer solution. Biomarkers are endogenous molecules, making it difficult to find native matrix without measurable levels of analyte. Thus, a surrogate matrix is the practical alternative. Generating analyte-depleted matrix is another approach, when feasible. Sometimes, the corresponding matrix from another species can be used if there is no cross-reacting biomarker present. It is desirable for the analyst to obtain information about the components of the surrogate matrix for method adaption and troubleshooting, as well as to assess the stability of the analyte in this matrix.
In most cases where relative changes in the concentrations of biomarkers are being evaluated, the use of a substitute matrix for calibrators may be sufficient for data reliability, provided that parallelism with endogenous analyte has been evaluated in biological matrix.
Accuracy and precision of measurements
Initial accuracy and precision acceptance criteria for sample analysis can be set using spiked buffer QC samples. However, native or spiked MCs (MC pools) are preferred for evaluation of precision and relative accuracy during validation. Once the analyte concentration levels in MC pools have been established (after multiple runs, preferably involving multiple reagent lots), they can be used to set both accuracy and precision of quantification in routine testing, as well as facilitating trend analysis and monitoring of lot-to-lot consistency in assay performance.
Stability of analyte in biological matrix
Assessment of endogenous analyte stability is a complex undertaking. Spiked controls in substitute matrix may not be used for analyte stability evaluation. For this purpose, native matrix pools (or individual samples) are essential, preferably at two or more endogenous analyte concentration levels. If native matrix pools are all naturally low, additional spiked pools (to cover the higher end of the range) can be included in stability studies. Caution must be exercised with spiked matrix stability pools as analyte recovery may differ from native pools. Moreover, stability tests performed only with spiked pools may not necessarily be clinically meaningful. Thus, native matrix pools containing endogenous biomarker provide more reliable stability information.
Parallelism & minimum required dilution
Whenever possible three or more high-concentration endogenous biomarker matrix samples should be used to evaluate parallelism by measuring the analyte concentration at multiple dilutions [11,45,46]. Acceptable test results will demonstrate that the kit calibration curve, made using the substitute matrix and the recombinant reference standard, has the same response–concentration relationship as that of the endogenous biomarker in authentic biological matrix. A minimum required dilution, derived from evaluation of dilutional linearity, can also be applied to mitigate the matrix effect (reduce background and nonspecific binding). Once the minimum required dilution is determined, the selectivity test and standard curve adjustment can be performed to define the assay range. Attaining sufficient sensitivity from the assay can be challenging if the drug is expected to suppress the biomarker level.
LOD & LOQ
In drug development the most important measure of assay sensitivity in quantitative bioanalysis is the LLOQ; the lowest concentration of analyte that can be measured with an acceptable level of bias, precision and total error [6]. LOD is the lowest amount of analyte that can be statistically distinguished from zero, but it cannot be quantified with certainty [49]. Kit manufacturers often use LOD as a measure of sensitivity of the assay. In some cases knowledge of the LOD can be helpful in assessing the presence of a biomarker below the LLOQ levels [49,50]. However, for most clinical applications thorough evaluation and definition of the assay LLOQ must be performed during assay selection and validation to confirm suitability of the assay for the intended application.
Ruggedness
Ruggedness is an important element of validation that includes pre-study and in-study performance monitoring components that last throughout the lifecycle of the method. Initial ruggedness of a kit-based assay can be documented primarily with the accuracy and precision data generated during the validation exercise by multiple analysts, on different days and using multiple lots of kits or reagents. Later, in service, trending of the results from QC or MC samples gives a fairly good indication of continuous rugged performance of the method. Anomalous trending profiles may be indicative of changes in the kit components and should alert the analyst to initiate a formal investigation and implement any necessary remedial actions to restore satisfactory assay performance.
Conclusion
There are challenges in using commercial kits for drug development ranging from management of kit lot changes over time to ensuring the kit selected is appropriate for the intended use. The authors have addressed categories of various commercial kits. An understanding of their inherent differences allows the user to identify suitable kits for feasibility evaluation and then to adapt and validate the chosen assay to meet the intended purpose. Although some categories of kits may be extremely well controlled during manufacture, minimizing the effects of lot changes, the application of any kit for use in drug development will usually require the user to rigorously assess its performance and suitability. Recommendations provided herein, for approaches to kit selection, adaptation and method validation should aid the standardization and harmonization of commercial kit applications in biopharmaceutical development. Additionally, such systematic approaches should facilitate the generation of high-quality data and more easily enable the user to meet reasonable expectations of internal stakeholders and regulators. Furthermore, owing to the challenges highlighted here, there is a strong desire for more collaborative efforts among kit users in the pharmaceutical industry and manufacturers leading to the design and marketing of more user-friendly products. 'Pharmagrade' kits with key characteristics, such as high inter-lot consistency, access to bulk reagents, analytical component documentation and standardized validation practices [51], will increase reproducibility and decrease the need for extensive end-user work, thus enabling more efficient use in the drug development sphere.
| In vitro diagnostic kits | Research use only kits | |
|---|---|---|
| Intended use | Diagnostic patient care | Research use |
| Regulatory compliance | US FDA – 21 CFR Part 820/510(k) clearance | Not regulated |
| Assay characterization guidance | CLSI Guidelines/CLIA | No guidance, manufacturer-dependent specifications |
| Documentation | Well documented | Kit inserts with variable degrees of information |
| Kit format | Multiple | Multiple |
| Sample matrix | Restricted to approved uses (e.g., human plasma/serum/urine) | Wide variety (e.g., animal/human fluids, tissues, culture media) |
| Fit-for-purpose adaptability | Closed system/dedicated instrument restricts adaptability | Open system, easily adaptable. Assay modification possible, requires analytical validation |
| Technical support | Support readily available | Extent of support varies from vendor to vendor |
| RUO kit attributes | Recommended adaptations | |
|---|---|---|
| Kit components† | ||
| Calibrators | Four or more levels provided in the kit | 6–7 levels preferred |
| Reference material | Typically recombinant protein provided in small quantities | Procure additional recombinant protein preferably from kit manufacturer |
| Controls | Two levels in proprietary matrix (or none) | Generate controls at 3–5 levels |
| Capture antibody | Capture antibody-coated plate (or antibody solution for coating) | |
| Detector system | Detector/reporter system (e.g., enzyme–Ab, biotin–Ab/streptavidin–enzyme conjugate, enzyme substrate) | |
| Wash buffer | Concentrated wash buffer (not sufficient if plate washer is used) | Procure additional wash buffer for use of plate washer |
| Assay characteristics | ||
| Parameters used | Variable from vendor to vendor | End-user evaluation |
| Calibration curve | Graphic representation of calibration curve (may not represent the curve specific for the assay) | End-user evaluation |
| Curve fitting algorithm | Often not specified or validated. Limited advice provided for fitting linear or non-linear curve only | End-user evaluation |
| Precision of measurements | Typically 2–3 samples of known concentration tested multiple times on one plate for intra-assay precision | Use surrogate matrix controls at 3–5 levels |
| Typically 2–3 samples of known concentration tested in multiple assays for inter-assay precision | Use matrix controls with endogenous analyte at 2 or more levels | |
| Recovery (or accuracy) | Average % recovery values (with high and low ranges) are provided | End-user evaluation |
| Linearity of dilution | Average % recovery values (with high and low ranges) are provided at dilutions tested | End-user evaluation |
| Parallelism | Generally not provided | End-user evaluation |
| Assay sensitivity | LOD typically defined as concentration corresponding to mean signal value+2x SD determined from 20 replicates of zero standard | End-user evaluation of LLOQ |
| Selectivity/matrix interference | Generally not provided | End-user evaluation |
| Antibody specificity/cross-reactivity | A list of analytes with statement that no significant cross-reactivity or interference was observed | End-user evaluation as needed |
| Reference standard characteristics | Concentration of reference standard is provided without indication of purity or stability | Evaluate stability as needed. Consider additional source of reference material |
| Analyte levels in normal and disease population | Information is not always supplied | End-user evaluation as needed |
| Documentation | Kit insert listing the assay components, describing the assay procedure and summarizing variable levels of assay characterization | Appropriate documentation as needed |
| Tasks | Exploratory method validation | Advanced method validation |
|---|---|---|
| Performance parameters | ||
| Calibrators | May need to use additional calibrators and anchor points | |
| ≥3 analytical runs | ||
| Selectivity | Perform if not done in feasibility assessment | Spike recovery of ≥10 lots each of matrix from normal and patient populations |
| Dilution linearity | Confirm | Confirm |
| Parallelism | Confirm | Confirm |
| Accuracy and precision | ||
| VS or QC samples | Typically surrogate matrix | Surrogate or biological matrix |
| VS/QC at ≥5 levels | VS/QC at ≥5 levels | |
| MC | Two pools (high and low) | 2 or more pools |
| Accuracy and precision | ≥3 analytical runs, including MC | ≥6 analytical runs, including MC |
| Stability | ||
| Reagent stability | Optional | Confirm according to validation protocol |
| QC storage stability | As needed (usually prepared fresh) | As needed (usually prepared fresh) |
| MC storage stability | Confirm with MC | Confirm with MC |
| Method robustness | ||
| Lot-to-lot variability | As needed | Test with MC on ≥3 lots if available |
| Analyst, instrument, and site variations | As needed | Multiple analysts, instruments, sites |
| Documentation | ||
| Validation plan/validation report | Recommended† | Required |
| QA audited documentation | Intended use-dependent | Required |
Key Terms
Critical Path Initiative: US FDA initiative to enable increased efficiency of clinical trials that, among other things, is intended to increase opportunities to understand the potential utility of biomarkers.
Inter-lot variability: In the context of this paper, the variability between different lots of reagents and kits.
Fit-for-purpose method validation: Refers to the concept that analytical validation needs to be tailored to intended use in terms of matrix, study population(s), stage of project and most critical utility of data for project decisions.
Research use only kits: Default labeling for all biomarker kits indicating that there is no regulatory approval endorsing the use of this kit in any application.
Key Term
Analytical method qualification: Generally refers to a streamlined form of analytical method validation in support of (mostly) non-GLP studies. Typically indicates reduced scope and sample size of analytical experiments used to define assay performance prior to sample analysis.
Key Term
Ligand-binding assay: An assay in which one or more key steps involves non-covalent binding interactions between a ligand (analyte) and a binding molecule (e.g., antibody or receptor). Immunoassays are a subset of this class of assays.
Key Term
Method adaptation: Alterations to a kit protocol to enable specific use. Ranges from addition of more calibrators and quality controls to changes in matrix, incubation times and detection systems.
Acknowledgements
The authors wish to acknowledge the support and advice from members of Immunoassay Biomarker Discussion Group within the Ligand Binding Assay Bioanalytical Focus Group, BIOTEC Section of the American Association of Pharmaceutical Scientists.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1 Health Care and Education Reconciliation Act of 2010. www.gpo.gov/fdsys/pkg/PLAW-111publ152/pdf/PLAW-111publ152.pdf
- 2 Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm077262.htm
- 3 US Department of Health and Human Services: Food and Drug Administration. Critical Path Opportunities List. www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/UCM077258.pdf
- 4 . The FDA Critical Path Initiative and its influence on new drug development. Ann. Rev. Med. 59, 1–12 (2008).
- 5 How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat. Rev. Drug Discov. 9(3), 203–214 (2010).
- 6 Fit-for-purpose method development and validation for successful biomarker measurement. Pharm. Res. 23(2), 312–328 (2006).
- 7 . Systematic analytical validation of commercial kits for the determination of novel biomarkers for clinical drug development. Bioanalysis 2(2), 237–247 (2010).
- 8 . Insights in the application of research-grade diagnostic kits for biomarker assessments in support of clinical drug development: bioanalysis of circulating concentrations of soluble receptor activator of nuclear factor κβ ligand. J. Pharm. Biomed. Anal. 48(5), 1282–1289 (2008).
- 9 . Commutability limitations influence quality control results with different reagent lots. Clin. Chem. 57(1), 76–83 (2011).
- 10 . Commercial immunoassays in biomarkers studies: researchers beware. Clin. Chem. 58(10), 1387–1388 (2012).
- 11 . The use of commercial assay kits for PK/PD analysis in drug development. In: Ligand-Binding Assays: Development, Validation and Implementation in the Drug Development Arena. Khan MN, Findlay JW (Eds). Wiley, NY, USA (2010).
- 12 Draft Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). (2013). www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf
- 13 US Food and Drug Administration. Overview of IVD Regulation. www.fda.gov/medicaldevices/deviceregulationandguidance/ivdregulatoryassistance/ucm123682.htm
- 14 IVD Guidance: Research Use Only products. A Guide for Manufacturers and Notified Bodies. (2004). http://ec.europa.eu/health/medical-devices/files/meddev/2_14_2_research_only_product_en.pdf
- 15 CE Marketing. http://en.wikipedia.org/wiki/CE_marking
- 16 Draft Guidance for Industry and Food and Drug Administration Staff. Commercially Distributed In vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Frequently Asked Questions. U.S. Department of Health and Human Services: Food and Drug Administration. (2011). www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM257460.pdf
- 17 Guidance for Industry and Food and Drug Administration Staff. Distribution of In vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only. U.S. Department of Health and Human Services: Food and Drug Administration. (2013). www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253307.htm
- 18 U.S. Department of Health and Human Services: Food and Drug Administration. Clinical Laboratory Improvement Amendments (CLIA). www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm124105.htm
- 19 . “Research Use Only” Reagents: is there an imperative for increased FDA oversight? Clin. Chem. 57(12), 1681–1683 (2011).
- 20 . Validation of immunoassay for protein biomarkers: bioanalytical study plan implementation to support pre-clinical and clinical studies. J. Pharm. Biomed. Anal. 55(5), 869–877 (2011).
- 21 Increased concentrations of soluble CD40 ligand, RANTES and GRO-alpha in preeclampsia – possible role of platelet activation. Thromb. Haemost. 86(5), 1272–1276 (2001).
- 22 Application of Biochemical Markers of Bone Turnover in the assessment and Monitoring of Bone Diseases; Approved Guideline; NCCLS document C48-A (ISBN 1–56238–56539–539).Vol. 24(No 22), (2004).
- 23 Pre-analytical effects of blood sampling and handling in quantitative immunoassays for rheumatoid arthritis. J. Immunol Methods. 378(1–2), 72–80 (2012).
- 24 Tartrate-resistant acid phosphatase (TRACP 5b): a biomarker of bone resorption rate in support of drug development: modification, validation and application of the BoneTRAP kit assay. J. Pharm. Biomed. Anal. 49(5), 1203–1212 (2009).
- 25 . Risks in biomarker analysis resulting in unfit data use in clinical drug development. Biomark. Med. 3(2), 103–107 (2009).
- 26 . When do you need a validated assay? Bioanalysis 3(24), 2729–2730 (2011).
- 27 Biomarkers Task Force of the NCI Investigational Drug Steering Committee. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 16(6), 1745–1755 (2010).
- 28 What Is Test Method Qualification? Proceedings of the WCBP CMC Strategy Forum. BioProcess Int. 2(8), 32–46 (2004).
- 29 . Adaptation of fit-for-purpose biomarker assay validation using commercial kits: a CRO perspective. Drug Research. 9, 33–34 (2010).
- 30 Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat. Biotechnol. 28(5), 455–462 (2010).
- 31 Nonclinical safety biomarkers of drug-induced vascular injury: current status and blueprint for the future. Toxicol. Pathol. 42(4), 635–657 (2014).
- 32 Failure of current laboratory protocols to detect lot-to-lot reagent differences: findings and possible solutions. Clin. Chem. 59(8), 1187–1194 (2013).
- 33 . More on lot-to-lot changes. Clin. Chem. 60(2), 413–414 (2014).
- 34 . Statement of concern about a commercial assay used to measure soluble hemojuvelin in humans. Am. J. Nephrol. 36(4), 332–333 (2012).
- 35 False biomarker discovery due to reactivity of a commercial ELISA for CUZD1 with cancer antigen CA125. Clin. Chem. 60(2), 381–388 (2014).
- 36 . The Predictive Safety Testing Consortium: a synthesis of the goals, challenges and accomplishments of the Critical Path. Drug Discov. Today Technol. 4(2), 47–50 (2007).
- 37 The Biomarkers Consortium: practice and pitfalls of open-source precompetitive collaboration. Clin. Pharmacol. Ther. 87(5), 539–542 (2010).
- 38 . Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 28(5), 436–440 (2010).
- 39 Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 21(6), 1249–1273 (2000).
- 40 Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm. Res. 20(11), 1885–1900 (2003).
- 41 . Appropriate calibration curve fitting in ligand binding assays. AAPS J. 9(2), E260–E267 (2007).
- 42 Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 24(10), 1962–1973 (2007).
- 43 . Biomarker method validation in anticancer drug development. Br. J. Pharmacol. 153(4), 646–656 (2008).
- 44 “Fit-for-purpose” method validation and application of a biomarker (C-terminal telopeptides of type 1 collagen) in denosumab clinical studies. AAPS J. 11(2), 385–394 (2009).
- 45 Ligand binding assay critical reagents and their stability: recommendations and best practices from the Global Bioanalysis Consortium Harmonization team. AAPS J. 16(3), 504–515 (2014).
- 46 . Development and validation of ligand binding assays for biomarkers. In: Ligand-Binding Assays: Development, Validation and Implementation in the Drug Development Arena. Khan MN, Findlay JW (Eds). Wiley, NY, USA (2010).
- 47 . Combined Shewhart–Cusum control chart for improved quality control in clinical chemistry. Clin. Chem. 23(10), 1881–1887 (1977).
- 48 Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J. Pharm. Biomed. Anal. 46(1), 18–29 (2008).
- 49 . Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 29(Suppl. 1), S49–S52 (2008).
- 50 . Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin. Proteomics 10(1), 13 (2013).
- 51 . Application of commercial research-grade biomarker assays in drug development: is it time to create ‘pharmaceutical-grade’ kits? Bioanalysis 4(20), 2427–2430 (2012).










