Endo-Porter–mediated delivery of phosphorodiamidate morpholino oligos (PMOs) in erythrocyte suspension cultures from Cope's gray treefrog Hyla chrysoscelis
Abstract
Cope's gray treefrog, Hyla chrysoscelis, is a freeze-tolerant anuran that accumulates cryoprotective glycerol during cold acclimation. H. chrysoscelis erythrocytes express the aquaglyceroporin HC-3, which facilitates transmembrane glycerol and water movement. Aquaglyceroporins have no pharmacological inhibitors, and no genetic knockout tools currently exist for H. chrysoscelis. A phosphorodiamidate morpholino oligo (PMO)–mediated expression knockdown approach was therefore pursued to provide a model for testing the role of HC-3. We describe a novel procedure optimized for specific, efficient knockdown of HC-3 expression in amphibian erythrocyte suspensions cultured at nonmammalian physiological temperatures using Endo-Porter. Our protocol includes three critical components: pre-incubation at 37°C, two rounds of Endo-Porter and HC-3 PMO administration at ~23°C, and continuous shaking at 190 rpm. This combination of steps resulted in 94% reduction in HC-3 protein expression (Western blot), substantial decrease in HC-3 expression in >65% of erythrocytes, and no detectable expression in an additional 30% of cells (immunocytochemistry).
Cope's gray treefrog, Hyla chrysoscelis, is a freeze-tolerant amphibian that accumulates cryoprotective glycerol during cold acclimation (1,2). Control of intracellular and extracellular water content, and glycerol distribution, promotes extracellular freezing while protecting intracellular structures from ice damage. Regulation of those water and glycerol fluxes is enhanced by aquaporins (AQPs) and aquaglycer-oporins (GLPs), integral membrane proteins that facilitate water and, in some cases, glycerol flux across cell membranes (3). Of special interest to the physiology of freeze tolerance is the GLP HC-3, cloned and functionally characterized from H. chrysoscelis (2). HC-3 is expressed in erythrocytes and other tissues (2,4,5), and HC-3 protein abundance in erythrocytes increases during cold acclimation (5). Although previous studies have shown a correlation between enhanced GLP expression, cryoprotection, and freeze tolerance (2,5–8), the functional role of these proteins in conferring freeze tolerance remains poorly understood. To address this question, an in vitro cell culture model system of HC-3–expressing cells was developed. Suspension cultures of erythrocytes from H. chrysoscelis were chosen because they are nucleated, metabolically active, and therefore capable of regulating gene expression as well as peptide-mediated endocytosis, and can be repetitively harvested as a highly homogenous cell population. Since there are no specific inhibitors for AQPs/GLPs, and tools for gene knockout technology do not exist for H. chrysoscelis, an antisense phosphorodiamidate morpholino (PMO) knockdown approach was pursued. Antisense PMOs act on R NA targets by sterically blocking the translation initiation complex (9). Techniques available for delivery of PMOs to cells in culture include syringe or scrape loading, lipsomes, cationic polymers, free uptake from the media, and electroporation (9). Electroporation has also been used in adult axolotl for studying tissue regeneration (10), whereas microinjection and electroporation are widely used for delivery of PMOs into nonmammalian developmental models including sea urchin, Xenopus, zebrafish, and Drosophila embryos (11). Endo-Porter (GeneTools, Philmoth, OR, USA) is a novel peptide that mediates PMO delivery through nonspecific endocytosis, is effective in a wide variety of cell types, is easy to use, and is nontoxic to cells (12). Endo-Porter has been used in vitro to deliver PMOs to mammalian adherent and nonadherent cells cultured at 37°C (12,13) and ex vivo in tissue explants (14), resulting in a range of 50–80% targeted expression knockdown. One recent study used Endo-Porter–mediated PMO delivery to target endogenous gene knockdown in cultured carp macrophages (15). Endo-Porter has also been used successfully to deliver PMOs in vivo in newts (16). To date, however, Endo-Porter has not been widely used in nonmammalian cell culture systems. The objective of this study was to determine under what conditions and with what efficiency, Endo-Porter–mediated PMO delivery could be used to knock down targeted gene expression in nonmammalian cell suspension cultures, specifically in amphibian erythrocytes.
The HC-3 PMO was selected using the criteria defined for custom PMO design available through GeneTools (GeneTools). The HC-3 antisense PMO sequence (5′-CCCATGTTGCTGAGCCTCTAGGTC-3′) was designed based on complementarity to the following region in HC-3 (indicated in brackets on the sense strand, with the start codon in parentheses): ACTACTCAGCCGGCAGCATCACAGCTCTCCCC[GACCTAGAGGCTCAGCAAC(ATG)GG]GCGCCAGAAGGAGGTTCTCA. The commercially available standard control oligo (5′-CCTCTTACCTCAGTTA-CAATTTATA-3′; Gene Tools) with no known target in H. chrysoscelis was used as a PMO control.
The optimized procedure is as follows: Whole blood from H. chrysoscelis was collected in heparinized capillary tubes, transferred to 15 -mL conical tubes containing 5 mL complete cell culture media [CCCM; 250 mOsM, RPMI 1640 medium supplemented with L-glutamine, 100 units/mL penicillin and streptomycin, 0.25 µg/mL amphotericin B (Invitrogen, Carlsbad, CA, USA), and 5% FBS (Fisher Scientific, Hanover Park, IL, USA)], centrifuged at 1000× g for 10 min, and resuspended in CCCM. Approximately 2 × 106 cells were incubated in 200 µL media in round-bottom polypropylene tubes (Fisher Scientific) at 37°C for 2 h while shaking at 190 rpm on a titer plate shaker (Lab-Line Instruments Inc., Melrose Park, IL, USA).
The suspension volume was increased to 1 mL followed by addition of one of the following treatments: A, no treatment (control); B, 10 µM Endo-Porter alone (EP); C, 3 µM HC-3 PMO alone (HC-3 M); D, 10 µM Endo-Porter and 3 µM HC-3 PMO (EP + HC-3 M); and E, 10 µM Endo-Porter and 3 µM standard control PMO (EP + control M). Endo-Porter and standard or HC-3 PMO were sequentially added to the erythrocyte cultures. Cultures were maintained at 22–25°C within the range of basal body temperature of live treefrogs for 24 h while shaking. After 24 h, media was replaced with 200 µL fresh CCCM and erythrocytes were again incubated at 37°C for 2 h while shaking. At the end of 2 h, 800 µL CCCM was added to each culture. The relevant cultures were spiked with an additional 3 µM control or HC-3 PMO and/or 10 µM Endo-Porter, and maintained for an additional 24 h at room temperature after which the media was replaced; cells were cultured for a total of 76 h. Initial optimization experiments showed that cell viability at 76 h (~98%) remained unchanged between untreated erythrocytes and erythrocytes cultured in Endo-Porter alone (6–10 µM), HC-3 PMO alone (3–10 µM), or Endo-Porter and HC-3 PMO (each at 10 µM) (data not shown). At 76 h, total cellular proteins were isolated, size-fractionated by SDS-PAGE, and subjected to Western hybridization using a peptide-derived, monospecific rabbit polyclonal antibody raised against sixteen C-terminal amino acids of HC-3 (0.44 µg/mL), or mouse anti–β-Actin antibody, (diluted 1:5000; Sigma-Aldrich, St. Louis, MO, USA) followed by incubation with HRP-conjugated goat anti-rabbit secondary antibody (1:1000; Santa Cruz Biotech, Santa Cruz, CA, USA) or goat antimouse secondary antibody (1:1000; Santa Cruz Biotech) as previously described (5). Relative band intensities were determined by densitometry using Vision Works software on a BioSpectrum Imaging System (U V P, Upland, CA). Averaged HC-3 protein abundance (normalized to β-actin) was expressed as a percentage of the no-treatment control group (A).
HC-3 protein abundance was reduced by 94% in treated cultures (treatment D) and showed no change in control cultures (treatments A–C and E) after 76 h, compared with no-treatment controls (Figure 1, A and B), indicating that HC-3 knockdown was specific to the Endo-Porter plus HC-3 PMO treatment. Immunocytochemistry was performed (as previously described in Reference 5) on erythrocytes harvested at 76 h to examine the efficiency and degree of HC-3 knockdown at the cellular level (Figure 2). Treatment D (Figure 2D) showed an overall 75% reduction in HC-3 expression, assessed semiquantitatively from combined cellular fluorescence (corrected for cell number) of experimental relative to control groups (Figure 2, A–C and E). Moreover, ~30% of erythrocytes showed complete HC-3 knockdown as assessed by the absence of fluorescence (Figure 2D), while >65% of erythrocytes had substantial reduction in HC-3 expression compared with treatment controls (Figure 2, A–C and E). Pre-incubation of the primary antibody with the immunizing peptide resulted in no immunoreactive signal (Figure 1A and Figure 2F), indicating that the signal observed in panels A–E is immunospecific for the HC-3 antibody. Thus, a high efficiency (>95%) of complete or substantial HC-3 knockdown was achieved using this method.
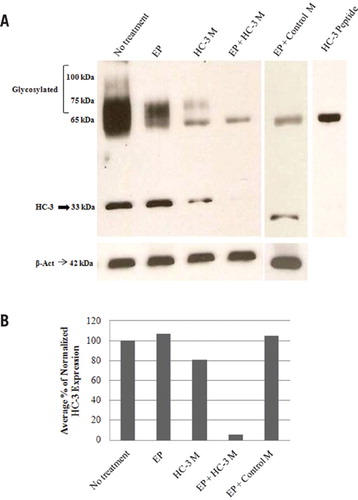
(A) Western blotting was performed as previously described (5), on total cellular proteins isolated after 76 h from erythrocytes subjected to the following culture treatment combinations: Lane 1, no treatment (control); Lane 2, 10 µM Endo-Porter alone (EP); Lane 3, 3 µM HC-3 PMO alone (HC-3 M); Lane 4, 10 µM Endo-Porter and 3µM HC-3 PMO (EP + HC-3 M); Lane 5, 10 µM Endo-Porter and 3 µM standard control PMO (EP + control M). An affinity purified peptide-derived antirabbit polyclonal antibody directed against the final 16 amino acids of the C-terminus of the predicted H. chrysoscelis HC-3 protein (previously described in Reference 5) was used to detect native and glycosylated HC-3 protein at 33 kDa (arrow) and >65 kDa respectively. Lane 6, Pre-absorption control. Pre-incubation of the HC-3 polyclonal antibody with 200-fold excess of the immunizing peptide blocked HC-3–immunospecific reactivity at 33 kDa and >65 kDa. A nonspecific band at 65 kDa not blocked by antibody pre-absorption with the immunizing peptide appears in all lanes. Expression of the housekeeping gene β-actin (42 kDa) as determined by Western blotting with a mouse anti–β-actin antibody is shown as a gel loading control. (B) HC-3 and β-actin expression was quantified by densitometry. For each treatment combination, HC-3 protein expression (normalized to β-actin) is expressed as a relative percentage versus normalized HC-3 expression in erythrocytes with no treatment. HC-3 protein expression in treated cultures (EP + HC-3 M) is reduced by an average of 94% with a range of 92–96% (n = 4).
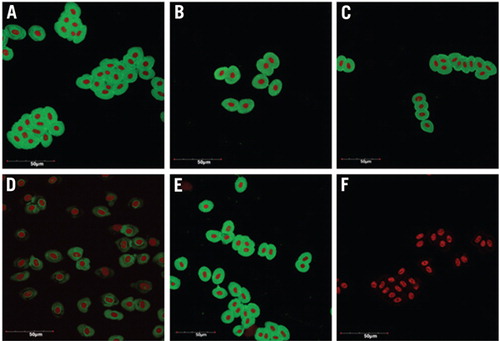
Immuncytochemistry was performed on cultured erythrocytes using a primary peptide-derived polyclonal antibody directed against the C-terminus of H. chrysoscelis HC-3. (A) No treatment (control). (B) 10 µM Endo-Porter alone (EP). (C) 3 µM HC-3 PMO alone (HC-3 M). (D) 10 µM Endo-Porter and 3µM HC-3 PMO (EP + HC-3 M). (E) 10 µM Endo-Porter and 3 µM standard control PMO (EP + control M). (F) Pre-absorption control. HC-3 expression is shown as a green signal due to the use of a fluorescein labeled secondary anti-body. Nuclei appear red after RNase treatment and propidium iodide staining. Approximately 30% of erythrocytes treated with EP + HC-3 M (panel D) showed complete HC-3 knockdown as assessed by the absence of fluorescence while >65% of erythrocytes had substantial reduction in HC-3 expression compared with treatment controls (Panels A–C and E) (n = 3 independent trials for each condition). No HC-3 signal (green) was seen in the pre-absorption controls (Panel F), where the HC-3 primary antibody was pre-incubated with 200-fold excess of the antigenic peptide, indicating that the HC-3 immunoreactivity seen in panels A–E is specific for the HC-3 antibody. Images were taken using an Olympus FluoView 1000 laser scanning confocal microscope (Center Valley, PA, USA) with a 60× objective. The scale bar indicates 50 µm.
The optimized procedure described in this report is novel in three important ways. First, cell viability and expression knockdown was dependent on shaking the cultures at 190 rpm throughout the procedure. Although the manufacturer recommends immediate swirling after Endo-Porter administration, it is likely that continual shaking throughout the incubation period contributed to the high efficiency of Endo-Porter mediated PMO delivery by increasing cell exposure to these reagents. Thus, continual shaking of adherent as well as nonadherent cell cultures from any source (mammalian and nonmammalian) may also result in improved PMO-mediated expression knockdown through increased delivery efficiency. Second, while frog erythrocytes do possess endocytic capacity (17,18), initial attempts at administering Endo-Porter and HC-3 PMO to erythrocytes cultured at ~23°C (which is within the range of normal physiological temperature for treefrogs) failed to achieve successful HC-3 expression knockdown. Therefore, we implemented a pre-incubation step at 37°C prior to the administration of Endo-Porter and PMO in an attempt to recapitulate the kinetic and thermal conditions for which the delivery vehicle was created. In addition, this 37°C pre-incubation step likely functions to increase the membrane fluidity of the cells, thereby improving the efficiency of peptide-mediated endocytosis (19). Third, preliminary experimental trials indicated the need for a second round of Endo-Porter and PMO administration for efficient knockdown. This is consistent with prior studies (20) and with manufacturer's recommendations (personal communication). In the procedure used here, a second 37°C incubation step was added prior to a second PMO administration, thereby coupling the effects of second-round PMO exposure with improved membrane fluidity. The combination of all three optimization steps was required to achieve targeted gene knockdown in our system. By using this improved and optimized approach, we achieved >95% targeted expression knockdown.
In conclusion, a novel procedure for efficient Endo-Porter–mediated HC-3 PMO delivery and expression knockdown in cultured amphibian erythrocytes has been described. This model will be used in future experiments to determine HC-3 function in freeze tolerance. However, the utility of this optimized delivery method is not restricted to amphibian erythrocytes. This novel procedure is likely to be of special interest and utility to investigators using nonmammalian systems (e.g., Xenopus, zebrafish, sea urchin, fish, newt, axolotl) who may not have previously attempted to use Endo-Porter due to concerns about its efficacy at non-optimal temperatures. Moreover, the combination of optimized steps can be broadly applied to mammalian as well as nonmammalian systems in order to improve overall knockdown efficiency. Finally, this report demonstrates that Endo-Porter–mediated PMO expression knockdown can be optimized for use in models for which genetic knockout tools do not currently exist.
Acknowledgments
This research was supported in part by the National Science Foundation (NSF; grant no. IOB-0517301, to C.M.K. and D.L.G.) and a University of Dayton Graduate Summer Fellowship (to V.M.) All procedures involving live animals were carried out using protocols approved by the Wright State University Institutional Animal Care and Use Committee.
Competing interests
The authors declare no competing interests.
References
- 1. . 2003. Geographic variation in energy storage and physiological responses to freezing in the gray treefrogs Hyla versicolor and H. chrysoscelis. J. Exp. Biol. 206:2859–2867.
- 2. . 2007. Excretion and conservation of glycerol, and expression of aquaporins and glyceroporins, during cold acclimation in Cope's gray treefrog, Hyla chrysoscelis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R544–R555.
- 3. . 2007. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm. Genome 18:452–462.
- 4. . 2010. Expression and immunolocalization of aquaporins HC-1, -2, and -3 in Cope's gray treefrog, Hyla chrysoscelis. Comp. Biochem. Physiol A Mol. Integr. Physiol. 157:86–94.
- 5. . 2010. Glycerol uptake by erythrocytes from warm- and cold-acclimated Cope's gray treefrogs. J. Comp. Physiol. 180:1257–1265.
- 6. . 2002. Aquaporin expression correlates with freeze tolerance in baker's yeast and overexpression improves freeze tolerance in industrial strains. Appl. Environ. Microbiol. 68:5981–5989.
- 7. . 2002. Altering fish embryos with aquaporin-3: an essential step toward successful cryopreservation. Biol. Reprod. 67:961–966.
- 8. . 2003. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol. Reprod. 68:87–94.
- 9. . 2002. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 1:347–355.
- 10. . 2005. Quantitative evaluation of morpholino-mediated protein knockdown of GFP, MSX1, and PA X7 during tail regeneration in Ambystoma mexicanum. Dev. Dyn. 232:162–170.
- 11. . 2001. Morphant technology in model developmental systems. Genesis 30:89–93.
- 12. 2005. Endo-Porter: A novel reagent for safe, effective delivery of substances into cells. Ann. N. Y. Acad. Sci. 1058:62–75.
- 13. . 2009. MINA, an IL-4 repressor controls TH2 bias. Nat. Immunol. 10:872–879.
- 14. . 2008. The use of Endo-Porter to deliver morpholinos in kidney organ culture. Biotechniques 44:547–549.
- 15. . 2011. A novel soluble immune-type receptor (SITR) in teleost fish: Carp SITR is involved in the nitric oxide-mediated response to a protozoan parasite. PLoS One 6:e15986.
- 16. . 2006. The role of Pax-6 in lens regeneration. Proc. Natl. Acad. Sci. USA 130:14848–14853.
- 17. . 1983. Functional integrity of desensitized β-adrenergic receptors. J. Biol. Chem. 258:6410–6414.
- 18. . 1984. Morphologic demonstration of clathrin-coated pits in frog and turkey erythrocytes. Exp. Cell Res. 151:573–577.
- 19. . 2009. Plasma membrane fluidity affects transient immobilization of oxidized phospholipids in endocytotic sites for subsequent uptake. J. Biol. Chem. 284:2258–2265.
- 20. . 2007. A switch in numb isoforms is a critical step in cortical development. Dev. Dyn. 236:696–705.












