A novel method for genetic transformation of yeast cells using oligoelectrolyte polymeric nanoscale carriers
Abstract
The genetic transformation of target cells is a key tool in modern biological research, as well as in many gene therapy and biotechnology applications. Here we describe a new method for delivery of DNA into several industrially important species of yeast, including Saccharomyces cerevisiae. Our method is based on the use of a novel nanoscale oligoelectrolyte polymer possessing a comb-like structure as a carrier molecule. Direct comparisons to standard transformation methods clearly show that our approach: (i) yields two times more transformants of Hansenula polymorpha NCYC 495 compared to electroporation approaches and 15 times more transformants compared to lithium acetate protocols, as well as (ii) 5 times more Pichia pastoris GS115 transformants compared to electroporation and 79 times more transformants compared to lithium acetate. Taken together, these results clearly indicate genetic transformation of yeasts using oligoelectrolyte polymer carriers is a highly effective means of gene delivery.
We describe a new method for rapid and efficient delivery of DNA into several industrially important yeast species using a novel oligoelectrolyte nanoscale polymer possessing a comb-like structure as the carrier.
Yeast species have been used in a wide range of applications in modern biology, including in the production of various biological agents in industrial biotechnology, as experimental models for signaling processes taking place in higher eukaryotic cells, and as powerful model systems to characterize the molecular events associated with human mitochondrial and neurodegenerative diseases, including Alzheimer's, Huntington's, and Parkinson's (1). Delivery of DNA into yeast cells is the most critical, and limiting, step when it comes to the development of new strains to advance yeast-based research. Various approaches have been developed to facilitate delivery of nucleic acids into yeast cells, including lithium acetate (LiAc)-based methodologies, electroporation, gene gun transformation, protoplast transformation, and others (2–16). However, all these approaches present limitations, such as low delivery efficiency, high toxicity, durable consumption time, complexity of application, high cost of reagents and/or equipment. In addition, the two most commonly applied methods for DNA delivery into yeast cells – namely electroporation and LiAc chemical transformation are not efficient for some yeast species of high biotechnological interest (3, 9, 10, 16, 17). Yarrowia lipolitica, Dekkera bruxellensis (Brettanomyces bruxellensis), Phaffia rhodozyma (Xanthophyllomyces dendrorhous), Hansenula polymorpha, and Candida lipolitica are yeast species that possess high potential for biotechnology applications such as bio-ethanol production, yet present significant time, cost and efficiency challenges in their transformation with heterologous DNA using standard methods.
Significant progress in yeast transformation has been achieved through the use of specific nanoscale particles for DNA delivery (10, 18–21). Gene gun technology can be used to transform yeast species refractory to DNA delivery; however, this method requires expensive equipment, and is also both time consuming and known to provide relatively low transformation efficiency for some yeast species (6, 22–24).
Efficient yeast transformation methods are challenging due to the presence of the yeast cell wall, which composed mostly of mannose-containing proteins and glycans (25). Typical protocols for DNA delivery into yeast cells include time consuming and/or expensive steps to enzymatically remove cell wall components with lyticase or zymolyase; and chemical pretreatment of yeast cells with polyethylene glycol, lithium chloride, or thiol compounds (all these procedures make the cell wall leaky to macromolecules), or with 2-mercaptoethanol, dimethyl sulfoxide (DMSO), or dithiothreitol (DTT) (17, 26–34).
Polyethylene glycol (PEG) is the only polymeric agent presently used for yeast transformation with the LiAc-based method and protoplast transformation. PEG-based transformation of yeast protoplasts or spheroplasts where the cell wall has been removed is a time consuming and complicated method with irreproducible and often unsatisfactory results (33–36). Thus, the lack of convenient, efficient, and nontoxic method for DNA delivery remains one of the biggest challenges in yeast basic research and biotechnology.
Here, we propose a novel method for DNA delivery into yeast cells based on using a new nanoscale comb-like oligoelectrolyte polymer. This oligoelectrolyte polymer combines an anionic backbone with dimethyl aminoethyl methacrylate (DMAEM)-based side branches to enable effective DNA delivery into a variety of yeast species.
Materials and methods
Synthesis and characterization of comb-like oligoelectrolytes
The novel DNA carrier is a copolymer of a comb-like structure that combines an anionic type oligoelectrolyte chain, copolymer of vinyl acetate (VA), 5-(tertbutylperoxy)-5-methyl-1-hexen-3-yne (VEP), and maleic anhydride (MA), as a backbone and 1 to 3 grafted side chains, predominantly 2, of the cationic type, copolymer of DMAEM and VEP (Figure 1). The combination of these chains in carrier molecules provides tightly controlled solubility in a wide pH range, optimal surface activity, and the ability to form and stabilize nanoscale inter-polyelectrolyte complexes with DNA and their derivative water-based systems.
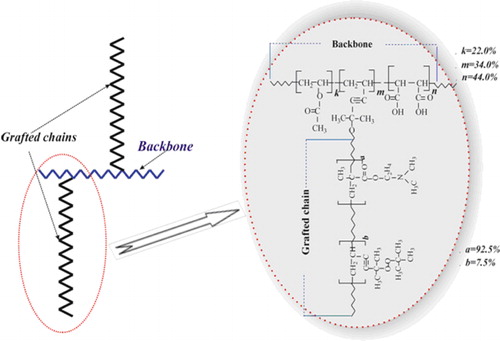
This comb-like polyampholitic polyelectrolyte was synthesized via controlled radical polymerization initiated by the oligoperoxide metal complex (OMC) in a polar organic media. OMC was coordinating Cu2+ complex of the copolymer composed of vinyl acetate, 5-tertbutylperoxy-5-methyl-1-hexene-3-yne, and maleic anhydride. Both the initial oligoperoxide and OMC derivate have been synthesized, as previously described (37–39).
Synthesis of the comb-like polyelectrolyte was carried out as follows: a monomer mixture of DMAEM (28.18 g (161.11 mol/l)) and VEP (3.19 g (15.31 mol/l)) was injected into round bottom glass reactor equipped with impeller mixer and backflow condenser. A solution of OMC (1.66 g (0.78 mol/l) in ethanol or dimethyl formamide (67 g (822.8 mol/l)) was then added with stirring. The incubation temperature was increased to 333 K, and the reactor was actively maintained at this temperature for 8 hrs under argon flow. The monomer conversion was controlled using a gravimetric technique; after achieving the desired degree of conversion (i.e., 60%), the solvent was evaporated under vacuum. Synthesized comb-like polyelectrolyte was purified by multiple precipitations from acetone solution into hexane, and dried under vacuum.
Synthesized polymer was dissolved in sterile distilled H2O, and the pH was adjusted to pH 7.4 (if not mentioned otherwise). A 1% water polymer solution was aliquoted into 1.5 mL plastic tubes (leaving as less air in the tube as possible), and stored at 4°C.
Yeast strains, growth conditions, and media
Hansenula polymorpha NCYC 495 leu1–1, Pichia pastoris GS115 his4 and Saccharomyces cerevisiae BY4742 MATα his3Δ leu2Δ lysΔ ura3Δ strains were grown in YPD medium (1% yeast extract (Serva, Germany), 2% bacto-peptone (Serva, Germany), 2% glucose (Serva, Germany)) at 37°C (H. polymorpha) or 30°C (P. pastoris, S. cerevisiae), correspondingly.
Minimal modified Burkholder medium was used for selection of P. pastoris and S. cerevisiae transformants (40). For the selection of H. polymorpha transformants via geneticin (G418) resistance, 50 mg/L of G418 (Invitrogen, Sweden) was added to YPD plates. For the auxotrophic strains, amino acids L-leucine, L-uracil, L-histidine, L-lysine (Sigma-Aldrich, USA) were added to a final concentration of 40 mg/L.
DNA manipulation and transformation of Escherichia coli were carried out according to standard procedures (41). E. coli strains were grown in Luria-Bertani medium (LB) at 37°C supplemented with ampicillin (100 µg/mL, Sigma-Aldrich, USA), if necessary.
EIectroporation assay
Electroporation was carried out by using a Bio-Rad Gene Pulser II, USA equipped with a Bio-Rad Pulse Controller II, USA. For yeast electroporation, a modified protocol from Becker and Guarente was used (4).
Transformants were counted 3–5 days after electroporation and statistical analysis (Student's t-test) was performed.
Lithium acetate transformation
A modified protocol for delivering DNA into yeast cells following treatment with lithium acetate (LiAc) was used (30). After 3–5 days of growth, yeast transformants were counted and statistical analysis (Student's t-test) was performed.
Yeast transformation using the developed polymer
H. polymorpha, P. pastoris and S. cerevisiae yeasts were grown overnight in non-selective YPD medium at 37°C (H. polymorpha) or 30°C (P. pastoris, S. cerevisiae). 150 µL of the overnight culture was transferred into 30 mL of YPD medium and grown until an OD600 ≤0.4–0.5. The cells were collected by centrifugation for 10 min at 3000 xg and then re-suspended in 100 µL of YPD. One µL of 1% water solution (pH 7.4) of oligoelectrolyte-based carrier BG-2 and 1 µg of plasmid DNA were added to the cell suspension, mixed gently and kept on ice for 45 min. Subsequently, cells were heat-shocked for 60–90 s at 42°C (H. polymorpha, S. cerevisiae) or 55°C (P. pastoris), chilled on ice for 2 min, and mixed with 1 mL of YPD medium. After incubation at 37°C (H. polymorpha) or 30°C (P. pastoris, S. cerevisiae) for 1 h, 100 µL of cells were plated on a selective medium and incubated at 37°C or 30°C, respectively. Yeast transformants were counted after 3–5 days of growth, and statistical analysis (Student's t-test) was applied.
Stable transformation of Hansenula polymorpha
Yeast transformants were obtained using the protocol employed for transient transformation. After recovering and counting of the transformants, cells were re-plated several times on either selective medium or non-selective medium and grown as described for transient transformation. Stably transformed clones were counted.
Statistical analysis
All experiments were repeated at least 3 times and in triplicate parallels. Results are presented as mean ± standard deviation. Statistical significance was evaluated by Student's t-test. Values of P<0.05 were considered statistically significant.
Results and discussion
We synthesized our DNA carriers by grafting side cationic (dimethyl aminoethyl methacrylate and 5-(tertbutylperoxy)-5-methyl-1-hexen-3-yne)containing branches to an anionic carboxyl-containing backbone chain (Figure 1;Table 1). The carriers were designed to contain positively charged polymer branches to enable binding of DNA via negative charges on nucleotide phosphates. A dynamic light scattering study (Supplementary Figure S1) showed that after encapsulation of plasmid DNA, particle size increased from 19.2 to 95.6 nm. Formation of a particle-DNA complex was also confirmed by measuring zeta potential, which increased from 30.6 (particle alone) to 35.4 mV (particle and DNA complex).
 |
The DNA binding capability of these carriers (Supplementary Figure S2) was evaluated by an electrophoretic method that monitors shifts in plasmid DNA band positions. The results of this electrophoretic analysis suggest formation of ion bonds between the polymer carrier and plasmid DNA. Polymeric carrier BG-2 exhibited the optimal set of indicators of DNA binding and yeast transformation, and was therefore selected for further use in this study.
For yeast transformation experiments, we used Hansenula polymorpha and Pichia pastoris which are known for high biotechnological potential, however, their genetic transformation shows low efficiency. We compared transformation efficiencies among three different methods: (i) the chemical-based LiAc method, (ii) electroporation, and (iii) the novel method we describe here based on using the oligoelectrolyte carrier.
Transformation of Hansenula polymorpha yeasts
Hansenula polymorpha is widely used in the production of bio-pharmaceuticals, genetically modified enzymes, vaccines, and other compounds of high industrial and medical importance (42–44). Although these yeasts exhibit many unique properties, their genetic engineering is limited due to low transformation efficiency when using the LiAc method (45). Therefore, electroporation is the most often used for genetic transformation of this yeast species, a technique that tends to be time consuming (necessitating competent cell preparation prior to electroporation), requires additional equipment, and frequently fails.
To test our new BG-2 polymer-based transformation method, we sought to transform the circular pGLG578 (contained LEU2 gene of Saccharomyces cerevisiae as a selectable marker) or linear HindIII-digested pYT3 (contained LEU2 gene of Saccharomyces cerevisiae as a selectable marker) plasmid (Institute of Cell Biology, National Academy of Sciences of Ukraine, Lviv, Ukraine) into H. polymorpha NCYC 495 leu1-1 yeast using the traditional LiAc method, electroporation, and the polymer-based technique. Leu+ and geneticineR transformants were selected on the YPD media supplemented with G418 (50 mg/L) antibiotic without leucine when pGLG578 plasmid was used. When pYT3 plasmid was used, Leu+ transformants were selected on solid minimal modified Burkholder media without leucine. The transformants were selected for leucine independence and G418 resistance.
Efficient transformation of this yeast strain using our method was usually achieved within 4 h, faster than either LiAc transformation (5 h) or electroporation (7 h). Additionally, our transformation protocol resulted in two times more transient transformants than electroporation, and 15.7 times more transformants than LiAc (Figure 2). It should be noted that both the electroporation and LiAc methods target only non-native cells that are made competent for transformation in a time consuming pre-treatment procedure. On the other hand, our method allows efficient transformation of native fast-growing log-phase yeast cells without any pre-treatments.

Lithium acetate method, electroporation, and BG-2 carrier-based transformation method were applied using the linearized pYT3 plasmid. Average number of transformants ± standard deviation is shown. ** - P <0.01.
Application of our method also enabled more efficient delivery of non-linearized plasmid DNA as compared with the above mentioned classic methods (Figure 3). In addition, we tested the different methods for stable genetic transformation of H. polymorpha NCYC 495 yeast. The use of our method resulted in the recovery of 170 stably transformed clones, comparing to 120 clones recovered when electroporation was used and 20 clones recovered from the LiAc method (Figure 4). All genetically transformed stable yeast clones were selected for leucine independence or/and for G418 resistance. A useful property for ascertaining that characteristic is an expression of a new phenotype that is caused by the plasmid presence. The presence of DNA insert has been confirmed by the PCR (data are not shown). The primers used in this study are listed in the Supplementary Table S1.

Chemical Lithium acetate method, electroporation, and BG-2 carrier-based transformation method were applied using the circular pGLG578 plasmid DNA. Yeasts were plated on a selective G-418-containing and leucine deficient medium. Average number of transformants ± standard deviation are presented. * - P <0.05, ** - P <0.01.

Chemical Lithium acetate method, electroporation, and BG-2 carrier-based transformation method were applied. Yeasts were plated on a selective G-418-containing and leucine deficient medium. Average number of transformants ± standard deviation is presented. * - P <0.05.
Transformation of Pichia pastoris yeast
Pichia pastoris is well known for its use in the biotechnology sector. It is one of the most attractive microorganisms currently used for the expression of heterologous eukaryotic proteins under the constitutive and inducible promoters (46–51).
Preliminary experiments indicated that we were able to transform P. pastoris GS115 his4 yeast with a pPIC3.5 (7.8 kb) plasmid DNA (containing HIS4 gene as a selectable marker) (Institute of Cell Biology, National Academy of Sciences of Ukraine, Lviv, Ukraine) under conditions shown previously to be optimal for H. polymorpha. His+ transformants of P. pastoris were selected on a solid minimal modified Burkholder medium without histidine. Transformants were then selected for histidine independence.
Although transformation was possible using these conditions, transformation efficiency with P. pastoris was not sufficiently high in these experiments. However, we found that elevation of the heat shock temperature from 42 °C to 55 °C led to a significant increase in genetic transformation of H. polymorpha.
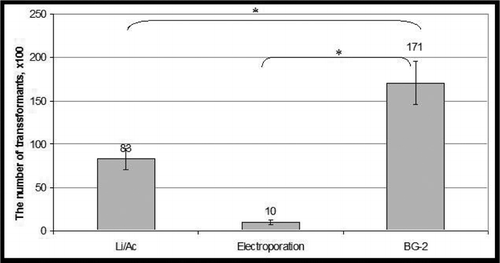
Thus, the method developed for genetic transformation of H. polymorpha and P. pastoris yeasts is more convenient, faster, and efficient than other currently used methods. This new transformation method resulted in five times more transient transformants than the electroporation, and 79 times more transformants than the LiAc method (Figure 5). Perhaps as important, our method of yeast transformation does not require the use of an electroporator or any other technical devices.

Lithium acetate method, electroporation, and BG-2 carrier-based transformation method were applied. Yeast was transformed using linear pPIC3.5 plasmid. Then yeast cells were plated on a solid Histidine deficient selective medium. Average number of transformants ± standard deviation is presented. *** - P <0.001.
Branched polyethylenimine (PEI) might be considered the closest commercially available analog to the polymeric carrier developed here. PEI has been widely used for DNA delivery in the mammalian cells (52–55). The mechanisms of action of the PEI, as well as our developed polymer, might be the following: (i) condensing DNA, (ii) providing protection against potential nuclease degradation, and (iii) promoting association with the plasma membrane of a target cell to enhance entry into the cell. Following an uptake, DNA should be capable of escape from the endosome and be released into the cytoplasm to be capable of reaching the nucleus. We found that PEI exhibited significantly lower transformation efficiencies with yeast cells, compared with our oligoelectrolyte polymeric nanoscale carriers.
Transformation of Saccharomyces cerevisiae yeast
Saccharomyces cerevisiae yeast is a unicellular eukaryotic organism most intensively used and studied in molecular and cell biology; in addition, it is widely used in biotechnology (6, 7, 16, 56). Although current transformation methodologies are enough sufficient for S. cerevisiae, we were interested to see if our oligoeletrolyte polymeric carriers could further enhance transformation efficiency.
Comparison of transformation efficiency using a circular episomal plasmid DNA (pYEp352 of 5.2 kb (containing URA3 gene as a selectable marker), Institute of Cell Biology, National Academy of Sciences of Ukraine, Lviv, Ukraine) and yeast Saccharomyces cerevisiae BY4742 MATα his3Δ leu2Δ lysΔ ura3Δ cells were performed. Ura+ transformants of S. cerevisiae was selected on a solid minimal modified Burkholder medium without uracil with transformants selected based on uracil independence.
Our polymeric carrier based transformation approach yielded 17,100 colonies of yeast transformants per 1 µg of DNA, while the standard electroporation protocol resulted in 1000 colonies, and chemical LiAc-based transformation yielded 8300 colonies (Figure 6).
Chemical Lithium acetate method, electroporation, and the BG-2 carrier-based transformation method were applied using circular pYEp352 plasmid. Yeast cells were plated on solid L-Uracil deficient selective medium. Average number of transformants ± standard deviation is presented. * - P <0.05.
Thus, there is no significant increase in the transformation efficiency when using our carrier with S. cerevisiae cells whose surface is well permeable to plasmid DNA. However, a distinct advantage in the transformation efficiency could be demonstrated when using yeast species such as Hansenula polymorpha and Pichia pastoris which have proven less emmenable to transformation in previous studies. These findings strongly suggest that the developed polymeric carrier possesses chemical characteristics necessary for efficient DNA delivery. We can hypothesize that such characteristics could include the hydrophobic core chain required for the improved penetration through the plasma membrane or possibly the positively charged polymer branches involved in binding DNA molecule, although this remains to be further investigated.
It is important to note that the polymeric carrier is highly efficiency for both transient and stable transformation when using either linearized or circular plasmid DNA. Moreover, there is no need for additional pretreatment steps to preparing competent cells or the requirement of special equipment for conducting the transformation. In addition, the developed method of yeast transformation is more convenient and rapid in use, and the proposed DNA carrier exhibits low toxicity and is not mutagenic (Supplementary Figures S3 and S4). This is unlike the widely used chemical-based LiAc method, in which costly geneticine antibiotic or other reagents are required for positive selection, and, thus, cells have to be incubated for long periods of time after transformation. Such prolonged cell incubation without selection makes experiments time consuming and decreases significantly the reproducibility of the results. A detailed comparison between the various transformation approaches can be found in Table 2.
 |
In conclusion, we have developed a novel method for efficient delivery of DNA into yeast cells using polymeric nanocomposites. We clearly demonstrated this method is more efficient and reproducible than other currently used methods for genetic transformation such as LiAc and electroporation. The developed method yielded a higher number of genetic transformants for the yeast species H. polymorpha and P. pastoris which are known to have a very low efficiency using current methods. Thus, our polymeric carrier represents the first nanoscale carrier for DNA delivery into the yeast cells, an approach that previously had only been applied for DNA delivery into the mammalian cells.
Acknowledgments
This work was partly supported by the grants from WUBMRC (Ukraine-USA), CRDF (USA), and F-46 project of the National Academy of Sciences of Ukraine, as well as by the project funded by the Ministry of Education and Science of Ukraine. The authors thank V. Nazarko, PhD, A. Polupanov, Y. Ryabec, PhD, I. Bohovych, and Y. Pynyaha, PhD (all from the Institute of Cell Biology, NAS of Ukraine) for their great help and fruitful discussions.
Competing interests
Authors declare no competing interests.
References
- 1. . 2005. Yeast as a model for medical and medicinal research. Trends Pharmacol. Sci. 26:265–273.
- 2. . 1990. Biolistic nuclear transformation of Saccharomyces cerevisiae and other fungi. Curr. Genet. 17:97–103.
- 3. . 2003. Gene gun Technologies: Applications for gene Therapy and Genetic Immunization. Gene and Cell Therapy 3:263–285.
- 4. . 1991. Highefficiency transformation of yeast by electroporation. Methods Enzymol. 194:182–187.
- 5. . 1995. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 254:241–263.
- 6. . 1996. Transformation of Saccharomyces cerevisiae mitochondria using the biolistic gun. Methods Enzymol. 264:265–278.
- 7. . 1998. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Technical Tips Online 1:P01525 http://tto.trends.com.
- 8. . 1997. Transformation system for a wastewater treatment yeast, Hansenula fabianii J640: isolation of the orotidine-5′-phosphate decarboxylase gene (URA3) and uracil auxotrophic mutants. Appl. Microbiol. Biotechnol. 48:621–625.
- 9. . 2001. Genetic transformation of yeast. Biotechniques 30:816–820.
- 10. . 2006. Transformation of yeast using bioactive beads with surface-immobilized yeast artificial chromosomes. Methods Mol. Biol. 349:61–65.
- 11. . 1982. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol. Gen. Genet. 187:326–329.
- 12. . 1982. A high-frequency transformation system for the yeast Kluyveromyces lactis. Curr. Genet. 6:123–128.
- 13. . 1978. Transformation of yeast. Proc. Natl. Acad. Sci. USA 75:1929–1933.
- 14. . 1986. Evidence for autonomous replication and stabilization of recombinant plasmids in the transformants of yeast Hansenula polymorpha. Curr. Genet. 10:741–747.
- 15. . 1979. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 76:1035–1039.
- 16. . 1991. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 7:691–692.
- 17. . 1986. Factors enhancing genetic transformation of intact yeast cells modify cell wall porosity. J. Gen. Microbiol. 132:3089–3093.
- 18. . 2002. Construction of YAC libraries with large inserts. Curr. Protoc. Hum. Genet. 5 Unit 5.2.
- 19. . 2009. Cationic polybutyl cyanoacrylate nanoparticles for DNA delivery. J. Biomed. Biotechnol. 2009:149254.
- 20. . 2010. Optimization of DNA delivery by three classes of hybrid nanoparticle/DNA complexes. J. Nanobiotechnology 8:6.
- 21. . 2011. Impedance Spectroscopy and FTIR Studies of PEG - Based Polymer Electrolytes. E-J. Chem. 8:347–353.
- 22. . 1988. Transformation of yeast by agitation with glass beads. Genetics 120:667–670.
- 23. . 2011. Nano-biolistics: a method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnol. 11:66.
- 24. . 2009. Transfection by particle bombardment: delivery of plasmid DNA into mammalian cells using gene gun. Biochim. Biophys. Acta 1790:754–764.
- 25. . 1984. Saccharomyces cerevisiace mannoproteins from an external cell wall layer that determines wall porosity. J. Bacteriol. 159:1018–1026.
- 26. . 1993. Dithiothreitol improves the efficiency of yeast transformation. Anal. Biochem. 208:211–212.
- 27. . 1981. Biofunctional change in yeast cell surface on treatment with Triton X-100. Agric. Biol. Chem. 45:2627–2629.
- 28. . 1992. Transformation of microbes, plants by particle bombardment. Biotechnology (N. Y.) 10:286–291.
- 29. . 1983. Transformation of yeast cells treated with 2-mercaptoethanol. Agric. Biol. Chem. 47:1691–1692.
- 30. . 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163–168.
- 31. . 1993. Introducing DNA into yeast by transformation. Methods 5:79–85.
- 32. . 1991. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 19:5791.
- 33. . 2000. Yeast transformation. In P.A. Norton and L.F. Steel (Eds.), Gene Transfer Methods: Introducing DNA into Living Cells and Organisms. Eaton Publishing, Natick, MA.
- 34. . 2002. Transformation of yeast by lithium acetate/single-stranded carrierDNA/polyethylene glycol method. Methods Enzymol. 350:87–96.
- 35. . 1983. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene 25:333–341.
- 36. . 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21:83–84.
- 37. . 1998. Kinetic features and molecular weight characteristics of terpolymerization products of the systems based on vinyl acetate and 5-tert-butyl-peroxy-5-methyl -1-hexene-3-yne. J. Appl. Polym. Sci. 67:1061–1066.
- 38. . 2001. Low-temperature surface-active complex-radical oligo (di-tert.alkyl) peroxide initiators and curing agents. Wiley-VCH 164:47–71.
- 39. . 2000. Surface-active metal-coordinated Oligoperoxidic Radical Initiators. J. Polym. Sci. A Polym. Chem. 38:516–527.
- 40. . 1978. Flavinogenic activity of natural strains of the yeast Pichia guilliermondii. Prikl. Biokhim. Mikrobiol. 14:184–189 in Russian.
- 41. . 2001. Molecular cloning, a laboratory manual, Cold Spring Harbor Laboratory, 3 ed. Cold Spring Harbor, N.Y.
- 42. . 2007. Isolation and characterization of mutated alcohol oxidases from the yeast Hansenula polymorpha with decreased affinity toward substrates and their use as selective elements of an amperometric biosensor. BMC Biotechnol. 7:33.
- 43. . 1992. Chromosomal targeting of replicating plasmids in the yeast Hansenula polymorpha. J. Gen. Microbiol. 138:2405–2416.
- 44. . 2007. Permeabilized cells of flavocytochrome b2 over-producing recombinant yeast Hansenula polymorpha as biological recognition element in amperometric lactate biosensors. Biosens. Bioelectron. 23:599–605.
- 45. 2002. Hansenula polymorpha: biology and applications, (WILEY-VCH – ISBN 3-527-30341-3).
- 46. . 1991. Yeast systems for the commercial production of heterologous proteins. Biotechnology (N. Y.) 9:1067–1072.
- 47. . 2003. Large scaleprotein production of the extracellular domain of the transforming growthfactor-type II receptor using the Pichia pastoris expression system. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 786:271–277.
- 48. . 2003. Membrane type-1 matrix metalloproteinase functions as a proproteinself-convertase. J. Biol. Chem. 278:8257–8260.
- 49. . 2001. Human insulin from a precursor overexpression in the methylortophic yeast Pichia pastors and a simple procedure for purifying the expression product. Biotechnol. Bioeng. 73:74–79.
- 50. . 2005. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 18:119–138.
- 51. . 2009. Genome sequence of the recombinant protein production host Pichia pastoris. Nat. Biotechnol. 27:561–566.
- 52. . 2008. Polyethyleneimine-mediated gene delivery into human adipose derived stem cells. Biomaterials 29:2415–2422.
- 53. . 2008. Polyethyleneimine-mediated gene delivery into rat pheochromocytoma PC-12 cells. J. Tissue Eng. Regen. Med. 2:288–295.
- 54. . 2009. Polyethleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 3:3273–3286.
- 55. . 2010. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small 6:239–246.
- 56. 1991. Guide to Yeast Genetics and Molecular Biology. Academic press Inc.












