A simple drying solution that minimizes cracking during air-drying of polyacrylamide gels
Abstract
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis is a routine technique used in biochemistry. Air-drying is an economical method of gel preservation that does not require expensive equipment. Our laboratory uses drying frames from RPI, which recommends a drying solution of 20% ethanol and 10% glycerol. The solution performs well for gels up to 10% acrylamide and 0.75 mm thickness; however, crack formation may occur if nicks or bubbles are present. The literature shows various drying methods and combinations of alcohol (30–100%) and glycerol (5–35%), but still reports cracking problems. Tests were conducted to independently evaluate the effects of ethanol and glycerol concentration on gel cracking. Here we introduce a simple solution that does not require glycerol or modified frames to generate preserved, crack-free sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels.
Method summary
In this study, several sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel-drying solutions containing varying amounts of glycerol and ethanol were used and compared. The gels were dried using cellophane sheets and drying frames, and then analyzed for crack formation. A simple solution containing only 40% ethanol avoids cracking reliably even in high-percentage PAGE gels.
Graphical abstract

Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) is a common procedure for protein analysis that separates proteins based on differences of molecular mass [1]. After this process, a Coomassie stain can be used to visualize the proteins in the gel slab. Gels are often preserved to keep in records as further evidence for the experiment. Air-drying and heat-drying are the most common methods for preserving gels. The heat-drying method usually involves heaters and vacuums that can be purchased, and although it is a relatively quick procedure, this method can be costly. Air-drying methods are a much more cost-effective way to preserve gels, but it takes a longer time for the gels to fully dry. The air-drying method usually involves a plastic frame and cellophane in which the gel is sandwiched.
Both the air-drying and vacuum methods still suffer the common problem of gel cracking. Gel cracking can occur when the drying process is too quick or when air bubbles get trapped near the gel. Our laboratory uses mini-gel-drying frames and film from RPI (Mount Prospect, IL, USA) for PAGE gel preservation. The manufacturer recommends a drying solution of 20% ethanol and 10% glycerol to minimize cracking [2]. This solution performs satisfactorily for gels up to 10% acrylamide and 0.75 mm thickness; however, care must be taken to avoid bubbles or nicking of gel edges, to prevent crack formation. Cracks seem to extend slowly even after the drying procedure is completed. Cracking occurs much more frequently with thicker gels (1.5 mm) or with gels of high acrylamide concentration (15% or more). We hypothesize that the solution used to dry the gel may play a role in increasing the likelihood of cracking. Several articles and protocols utilize varied solutions with different types of alcohols, but retain glycerol as a component of the drying solution [3–7]. We tested several drying solutions using varying amounts of glycerol while keeping the ethanol concentration constant, and vice versa.
Tests with varying concentrations of glycerol were first performed. The ethanol concentration was fixed at 20% while glycerol concentrations of 0, 10 and 40% were tested. For the test, 10% SDS-PAGE gels of 1.5 mm thickness were incubated in the drying solution for at least 1 h. A cut was made with scissors in the side of the gels as a seed for cracking before setting the gels in the drying frames for 24 h. The gels were set in the drying frames following the manufacturer’s recommendations (Tut’s Tomb Gel Dryers, RPI). Images of the drying frames utilized are provided in Supplementary Figure 1. Briefly, one wet sheet of cellophane was first placed on a solid square drying frame. The gel was transferred onto the wet cellophane sheet and a second sheet of wet cellophane was placed on top of the gel, creating a sandwich. Any trapped air bubbles were removed, and an open square frame was placed on top. The cellophane sheets were gently stretched to remove any wrinkles before the drying frame sandwich was secured by placing clamps all around it (Supplementary Figure 2 shows images of some of the gels set in the drying frames). Following the 24-h air-drying period, the gels were examined for cracks and removed from the drying frames. The gel that was dried with 40% glycerol showed no crack formation while in the frame, but upon removal it cracked severely within a few minutes (Figure 1A). In the gel dried with 0% glycerol, a crack extended from the cut while the gel was still in the frame (Figure 1C). Not only did the 10% glycerol gel show extended cracks from the cut while in the frame, but additional cracks appeared from the edges of the gel (these cracks also extended while the gel was stored in records; Figure 1B). Further testing was performed upon gel removal from the frames by nicking corners or edges with forceps (pinching). Nicking of the corners/edges by forceps pinching caused additional crack formation and extension in the 10% glycerol but not in the 0% glycerol gel.

A cut was made in the side of each gel to induce cracking, before placing the gels in the drying frames for 24 h. (A) The gel was dried using 40% glycerol and 20% ethanol as gel-drying solution. After removal from the drying frame, the gel began to crack severely. (B) The gel was dried with 10% glycerol and 20% ethanol as gel-drying solution. The gel cracked while in the frame, and the cracks extended while it was stored in records. (C) In the gel with 0% glycerol and 20% ethanol as its drying solution, a crack extended.
Tests with varying concentrations of ethanol were conducted next. Glycerol concentrations were fixed at 0% for these tests. Ethanol concentrations of 0, 20 and 40% were used with 10% SDS-PAGE gels of 1.5 mm thickness. The gels were incubated in the respective drying solution for 1 h before setting them in the drying frames. As was done with the glycerol concentration test, the gels were again cut on one side to facilitate cracking before being placed in the frames. The gels were allowed to dry for 24 h and checked for cracks before removing them from the frames. The gels dried in 0% ethanol (Figure 2A) and 20% ethanol (Figure 2B) showed cracking, while the gel dried in 40% ethanol (Figure 2C) did not. The cracks in the 0 and 20% ethanol gels did not extend further after the gels were removed from the frames. Pinching of the corners/edges with forceps after removal from the frames did not cause cracks to extend or new cracks to appear in any of the three gels. The drying procedure was repeated numerous times with the 40% ethanol solution to ensure no cracking would occur. None of the gels showed signs of cracking, confirming the results obtained.

As in Figure 1, the gels were cut on one side, to facilitate cracking, before being placed in the drying frames. (A) The gel was dried in a solution with both 0% ethanol and 0% glycerol (ddH2O was used as the gel-drying solution). A crack formed in the gel while the gel was in the drying frame. (B) The gel was dried using 20% ethanol and 0% glycerol as the gel-drying solution. A crack formed in the gel while the gel was in the frame. (C) This gel was placed in 40% ethanol as the gel-drying solution and did not form cracks while in the frame. The cracks in the 0 and 20% ethanol gels did not extend when the gels were removed from the frames. Pinching the corners or edges of the gels with forceps after removal from the frames did not cause crack extension or new cracks to appear in any of the three gels.
Tests were performed on 15% SDS-PAGE gels (with a thickness of 1.5 mm) due to their high risk of cracking. One gel was dried with a 40% ethanol solution while the other was placed in the previous drying solution of 10% glycerol and 20% ethanol recommended by the company. The gel dried using the 10% glycerol and 20% ethanol solution cracked severely upon removal from the frame, and the cracks extended further during storage (Figure 3A). The 40% ethanol with 0% glycerol gel showed no crack formation either before or after removal from the frame, or during storage in records (Figure 3B).

(A) A 15% PAGE gel was soaked in the company’s suggested drying solution of 10% glycerol and 20% ethanol. As observed in the figure, the gel cracked severely following removal from the frame. (B) The other 15% PAGE gel was soaked in a 0% glycerol and 40% ethanol drying solution. This gel did not crack after removal from the frame and remained intact while stored in records.
To confirm the reliability of the protocol and that no other variable was involved in the previously described experimental observations, an additional test was performed with 15% SDS-PAGE gels, also of 1.5 mm thickness. Three 15% SDS-PAGE gels were loaded with varying concentrations of the same set of proteins, and run and processed in parallel (under the same conditions). The gels were run at 80V for 3.5 h, stained with Coomassie Brilliant Blue overnight (15 h) and then destained for 2 h, with a solution change every 20 min. The gels were then placed for 1 h in their respective drying solutions before being set in the drying frames. They were observed every 24 h and removed from the frames at 72 h. Upon removal from the frames, the gels were pinched with forceps at both sides (a few mm from the bottom of the gel) to facilitate cracking. The gel dried in the 10% glycerol and 0% ethanol drying solution cracked immediately upon removal from the frame (Figure 4A). The gel dried in the company’s recommended solution (10% glycerol and 20% ethanol) took a few hours to crack, but it also cracked severely before 24 h had elapsed after frame removal (Figure 4B). The gel dried in 40% ethanol exhibited no cracking (even after pinching of the gel sides upon removal from the frame), and remained intact while stored in records (Figure 4C). Supplementary Figure 2 shows images of these three gels in the drying frames and at various times following removal from the frames. The dimensions of the gels were measured before and after the 1-h incubation in the respective drying solutions. All three gels had the same dimensions before being placed in the drying solutions, with a height of 5.7 cm and a width of 8.7 cm. After the 1-h incubation period in their respective drying solutions, the gel in 10% glycerol and 20% ethanol had a height of 5.2 cm and a width of 8.0 cm. The gel incubated in 10% glycerol expanded to a size of 6.1 cm (height) by 9.0 cm (width). The gel in 40% ethanol shrunk significantly, with a height of 4.5 cm and a width of 7.1 cm. This gel was completely dried after 24 h in the drying frame. However, the gels dried in the 10% glycerol and 20% ethanol solution and the 10% glycerol solution still seemed to retain moisture after 72 h in the drying frames.

Upon removal from the drying frames, the gels were pinched with forceps at both sides (near the bottom of the gel) to facilitate cracking. (A) The gel was dried using 10% glycerol as the drying solution. Immediately after removal from the drying frame, the gel began to crack severely (before pinching was performed) and continued cracking afterward. (B) The gel was dried with 10% glycerol and 20% ethanol as the gel-drying solution. The gel did not crack immediately upon removal from the frame or after pinching; however, it had also cracked severely within 24 h from frame removal. (C) The gel was dried using 40% ethanol. This gel did not crack before or after removal from the frame (even after pinching) and remained intact while stored in records. Additional images corresponding to this analysis are presented in Supplementary Figure 2.
A further test of the 40% ethanol drying solution was conducted with a larger (15.4 × 13.1 cm) 15% SDS-PAGE gel, also of 1.5 mm thickness. This gel was stained and destained as indicated above for the three mini-gels. The gel was incubated in the 40% ethanol drying solution for 2 h (with two solution changes, each after a period of 40 min) instead of 1 h (as done for the mini-gels), to ensure adequate solution equilibration, before being set in the drying frame. The gel was removed from the frame at 72 h. There were no signs of cracking before or after removal from the frame or during storage in records (Figure 5). This gel was also observed to be dried after 24 h in the frame and to shrink significantly during incubation in the drying solution (to 12.6 × 11.3 cm at the end of the 2-h incubation period).

A larger 15% SDS-PAGE gel (15.4 × 13.1 cm) was air-dried using 40% ethanol as the drying solution. The gel did not crack before or after removal from the drying frame, or during storage in records.
An additional test of the 40% ethanol drying solution was performed using non-denaturing gels (15% PAGE mini-gels, 1.5 mm thickness). The gels were stained and destained as indicated for the gels above, incubated in the 40% ethanol drying solution for 1 h and then placed into drying frames for 72 h. The gels were fully dried after 24 h in the frames, and they exhibited no cracks while in the frames, after removal from the frames, or during storage in records (Figure 6).
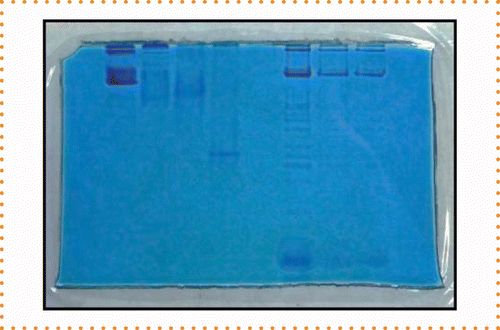
A 15% non-denaturing polyacrylamide gel electrophoresis mini-gel was dried using 40% ethanol. The gel did not crack before or after removal from the drying frame and remained intact while stored in records.
These tests concluded that using a drying solution of 0% glycerol and 40% ethanol appears to provide the optimal conditions to prevent cracking (probably by reducing the level of gel shrinkage during the drying procedure in the frames, given that the gels incubated in this drying solution shrink significantly before being set in the frames). It was observed in the tests that although glycerol kept the gels more moist and flexible, it did not prevent them from cracking. The tests performed showed that the gels crack more often as the concentration of glycerol in the drying solution increases, and that glycerol prolongs the period of crack growth beyond the gel-drying period. Gels dried using the new solution are less flexible but, importantly, they do not break under crack-inducing stimuli. Another benefit of this solution is that, because it does not contain glycerol, it reduces the length of time the gel needs to dry in the drying frames. We believe that this simple PAGE gel-drying solution may be useful as a reliable procedure for preserving intact dried gels for long-term storage.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/suppl/10.2144/btn-2022-0094
Author contributions
K Gomez, R Pérez-Ballestero, W Reddig, M González-García and A Patil participated in writing the manuscript. R Pérez-Ballestero, W Reddig, K Gomez and A Patil performed the gel-drying experimental procedures. J Alcala prepared lysates and purified proteins for PAGE. J Alcala, M González-García, R Pérez-Ballestero and A Patil obtained images, and A Patil prepared the figures for publication. M González-García and R Pérez-Ballestero designed, managed and supervised the project. All authors analyzed the results obtained, participated in manuscript review and editing, and approved the manuscript.
Acknowledgments
The authors would like to thank A Gonzalez for his help in editing the first draft, and J Gomez for her assistance in preparing solutions needed for some of the experiments performed.
Financial & competing interests disclosure
The authors would like to acknowledge the NIH-NIGMS-MBRS-SCORE-SC3 grant GM122615 to M González-García and R Pérez-Ballestero, and the Robert A Welch Foundation Grant AC-0006 to the Department of Chemistry at Texas A&M University-Kingsville, for support of this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. . SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal. Biochem. 87(2), 386–396 (1978).
- 2. Research Products International. Gel dryers-Tut's tomb. https://d2gdaxkudte5p.cloudfront.net/system/product_documents/Gel%20Dryers%20TDS_7.pdf
- 3. . A fast and inexpensive procedure for drying polyacrylamide gels. Anal Biochem. 287(1), 185–186 (2000).
- 4. . Gel drying methods. Methods Mol. Biol. 1853, 269–271(2018). • Describes the advantages of simple sodium dodecyl sulfate–polyacrylamide gel electrophoresis air-drying procedures.
- 5. . A simple method of drying polyacrylamide slab gels that eliminates cracking. Biotechniques. 70(1), 54–57 (2021). •• Describes a method to eliminate cracking by using a sieve acrylic plate to slow down the speed of gel dehydration; they use 30% methanol and 5% glycerol as the drying solution.
- 6. . SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) of proteins. Curr. Protocols Microbiol. 22, A.3M.1–A.3M.13 (2011).
- 7. . A simple and inexpensive method for drying high-percentage polyacrylamide gradient gels. Biotechniques. 8(4), 381–382 (1990). •• Describes a method using a solution with glycerol and a high concentration of ethanol for pre-shrinking high percentage acrylamide gels before air drying.













