The costs of using unauthenticated, over-passaged cell lines: how much more data do we need?
Abstract
Increasing data demonstrate that cellular cross-contamination, misidentified cell lines, and the use of cultures at high-passage levels contribute to the generation of erroneous and misleading results as well as wasted research funds. Contamination of cell lines by other lines has been recognized and documented back to the 1950s. Based on submissions to major cell repositories in the last decade, it is estimated that between 18% and 36% of cell lines may be contaminated or misidentified. More recently, problems surrounding practices of over-subculturing cells are being identified. As a result of selective pressures and genetic drift, cell lines, when kept in culture too long, exhibit reduced or altered key functions and often no longer represent reliable models of their original source material. A review of papers showing significant experimental variances between low- and high-passage cell culture numbers, as well as contaminated lines, makes a strong case for using verified, tested cell lines at low- or defined passage numbers. In the absence of cell culture guidelines, mandates from the National Institutes of Health (NIH) and other funding agencies or journal requirements, it becomes the responsibility of the scientific community to perform due diligence to ensure the integrity of cell cultures used in research.
Effects of Over-Subculturing
Scientific contributions from investigators using continuous cell lines as research tools are significant. However, the ability of continuous cell lines to exist almost indefinitely opens the possibility that cell lines are used beyond safe passage numbers (the point at which the cell culture no longer maintains key gene functions and consistent morphology). Divergent effects of long-term culturing on cell line morphology, development, and gene expression have been documented on key cell lines (1–6). Long-term subculturing places selective pressure on cell line traits leading to, for example, faster growing cells that eventually overrun slower proliferators in the population. In addition, cell lines maintained in culture over a long period of time may experience mutations that alter the original functional characteristics of the cell lines identified at earlier passage levels (6). When cell lines are obtained from colleagues, they often lack verification or documentation about the condition or passage number of the lines. This practice increases the likelihood that inferior, malperforming cultures are used, leading to results that may not be accurate or reproducible (7). Mossberg found that of 1402 surveyed scientists working in industrial settings, 69% reported obtaining one or more cell lines from another researcher or another source, and nearly half of the respondents reported never testing for identity with the lines they use (8).
Passage Number
The age of a tree can be determined by counting the rings in a cross-section of the trunk. A similarly straightforward method for determining the passage number of adherent cell lines does not exist. Stocks of adherent cell lines maintained in laboratories may differ by hundreds of passages. The impact of a cell line's age (or the number of times it has been passaged) on any given cell line is complex and dependent on several factors including the tissue and species of origin, the culture conditions, and the application for which the cells are used (4,9–12). Furthermore, cell lines do not behave similarly with increased passage number. For example, high-passage Caco-2 cells show an increase in the expression of the green fluorescent protein (GFP) reporter gene after transfection, while high-passage MCF7 cells exhibit a decrease in GFP expression (P. Ikonomi, manuscript in preparation). While observed effects appear at different degrees of subculturing, the potential consequences of using over-subcultured cell lines remain.
The human Caco-2 cell line has been the focus of several studies regarding the influence of passage number on several cell line-specific characteristics, including transport and toxicity of endogenous and exogenous compounds (1,13–15). The Caco-2 cell line is an established model of intestinal absorptive epithelium due to the ability of the cells to form a tight monolayer and to express key enterocytic markers and drug transport mechanisms upon differentiation. Reports demonstrate cell passage level can lead to variability in the key properties that define the ability of Caco-2 cells to predict drug absorption in vivo (Table 1).
 |
In 1996, Sun Lu et al. demonstrated passage-related differences in cell proliferation and transepithelial electrical resistance (TEER) linked to the tightness of the cell monolayer between early- (passage 35–47) and late-passage cells (passage 87–112). This indicates that as passage levels increase, a positive selection of faster-growing subpopulations of cells present in the heterogeneous parental line occurs, forming a tighter monolayer (13). This positive selection of subpopulations of cells was also shown by Briske-Anderson et al. in 1997 (1). In this report, the authors examined Caco-2 cells from passage 20 through passage 109 and found the TEER values increase up to passage 36 then decline after passage 60. They also found the proliferation rates of the cells and the activity of alkaline phosphatase increased in the later passage cells. Also in 1997, H. Yu et al. showed low- (passage 28–36) and high- (passage 93–108) passage Caco-2 cells differ in their compound transport characteristics. Cells at highpassage levels showed reduced carrier-mediated transport, reduced paracellular permeability, and increased transcellular permeability consistent with a reduction in the functional expression of a brush border enzyme and several transport proteins (6).
More recently, in 2005, Sambuy et al. examined the effect of cell-related factors on parental and clonally derived Caco-2 cell lines. This data supported the proposition that passage number influences brush border enzyme activities, morphology, TEER, proliferation rate, cell density, glucose transporter expression, carrier-mediated transport activities, and metabolic activities. It also highlighted the importance of culture conditions, such as seeding density, and medium composition, in influencing Caco-2 variability (5).
To investigate the impact these sources of variability have on the reproducibility of data from the Caco-2 cell model, LGC and ATCC, in a collaborative study with the National Institute for Biological Standards and Controls (NIBSC), investigated the effect of passage number, cell source, and medium composition on the transepithelial permeability, TEER, and proliferation rates of Caco-2 cells. This study observed a decrease in the TEER properties of cells passaged over 50 times, an increase in the paracellular permeability of the monolayer (consistent with the decreasing TEER values), a decrease in the transcellular permeability of the cells (consistent with a reduction in p-GP), and an increase in the proliferation rate of the cells as the passage number increased. These increases occurred in a number of different cell sources [ATCC, German Collection of Microorganisms and Cell Cultures (DSMZ), and European Collection of Cell Cultures (ECACC)] over the same passage range and were heavily influenced by the type of medium used to grow the cells (www.mfbprog.org.uk/publications/publications_item.asp?intPublicationID=1365).
The effect of extended passaging on cell culture characteristics is not limited to the Caco-2 cell model. In response to an increasing interest in agonists able to modulate growth and differentiation of prostate tumor cells, Esquenet et al. reported results of LNCaP prostatic adenocarcinoma cells derived from low-(passage 24–32) and high- (approximately passage 80) passage cells (3). This cell line, originally derived from a lymph node metastasis in 1977, has become an established model for the study of prostate cancer progression due to its proliferative and secretory responses to androgens. Esquenet et al. reported the high-passage cells showed divergent results compared with low-passage controls. The high-passage cells displayed an increased proliferative response when exposed to increasing concentrations of androgens, as well as a loss of the characteristic inhibition of growth in the presence of the synthetic androgen R1881. The high-passage cells also had a lower secretion rate of the differentiation marker prostate-specific antigen (3). These changes in cell line characteristics, due to increasing passage, are most likely attributable to genetic heterogeneity within the parental line, which leads to a positive selection process during prolonged cell culture (16,17).
In further studies into the effect of passage number, Wenger et al. reported results of a study comparing two sources (cell repositories and other laboratories) for each of the established cancer cell lines, MCF7 (breast cancer) and Ishikawa (endometrial tumor), which had all been subcultured for over 100 passages (7). All four cell line samples were examined by karyotype and comparative genomic hybridization (CGH). The authors found that the MCF7 cell lines showed slight karyotype differences (also observed by CGH analysis), suggesting genotype changes occurring over time due to cell passage level and maintenance in different laboratories. The Ishikawa cell lines showed no karyotype similarities other than a missing X chromosome with CGH analysis, suggesting that one or both of the lines became contaminated or that extensive passaging resulted in genetic drift. This study illustrates the changes a cell line may undergo due to multiple passaging and also emphasizes the importance of confirming the identity of cultured cells used for research, especially when obtained from a source other than a cell repository (7).
Cell line authentication is equally important with human embryonic stem (hES) cells, which are purported to have a limitless proliferation potential. A number of reports have been produced recently that highlight potential genetic frailty in hES cells, which can be exposed during the passaging procedure. Draper et al. reported the duplication of the long arm of chromosome 17 translocated to 6q in a subpopulation of the hES cell line H7 following 60 passages in culture (18). A similar result was reported by Inzunza et al. (19), who observed a normal chromosomal content in an hES cell line (HS237) at passage 35, but chromosomal aberrations in the X chromosome when the cell line was reanalyzed at passage 61. Mitalipova et al. (20) showed techniques used for cell passaging can also have an effect on the genetic stability of stem cells. They demonstrated that bulk passage methods after as few as 23 passages compared to manual passage methods, leads to aneuploidy detectable by karyotyping (20). Bulk passage methods include use of either enzymatic disaggregation (collagenase/trypsin) or nonenzyme-based methods using cell association buffer. Maitra et al. reported that following long-term culturing, a number of late-passage hES cell lines from different sources had at least one genetic abnormality commonly observed in human cancer cells, including DNA copy number and promoter methylation (21).
Passage number has also been shown to influence pluripotency of ES cells. Li et al. using the technique called tetraploid embryo complementation (where a mouse ES cell is aggregated with a four-cell-stage tetraploid embryo, then implanted, and allowed to develop in the uterus of a pseudopregnant female mouse), demonstrated that increased ES cell passage number negatively affected the ability of the cells to form an adult mouse (22). This technique is considered the most reliable method for determining ES cell pluripotency and demonstrates the important effects passaging can have on cells considered to have limitless prolif-erative potential.
Cross-Contamination and Misidentification
The problem of intraspecies and inter-species cross-contamination among cell lines has been recognized for half a century, and although reviews have been published, evidence of continued use of misidentification and cellular cross-contamination of cell cultures has not declined (23–36). Masters et al. found as much as 20% of all ostensible cell lines in use today are not as they are purported (37), and other results support these findings (23). An additional report estimates that more than one-third of cell cultures are cross-contaminated either with cells from other species (inter-species contamination) or with unrelated cells from the same species (intraspecies contamination) (32). MacLeod et al. (24) tested 252 human cell lines deposited at the DSMZ and found similar levels of cross-contamination; namely, 18% or 45 lines. The authors reported, “These misidentified cell lines have already been used in several hundreds of potentially misleading reports, including use as inappropriate tumor models and subclones masquerading as independent replicates” (24). A sample of known past cross-contamination reports is depicted in Table 2.
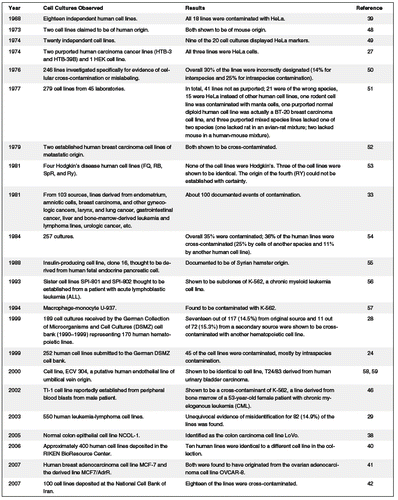 |
One of the earliest reports suggesting a high frequency of HeLa cell cross-contamination was made by Stanley Gartler in 1966 at a cell culture conference. Gartler later published in Nature, where it was shown that 18 lines thought to be of Caucasian tumor origins were found to contain isoenzyme A of G6PD, the same isoenzyme variant present in the aggressive HeLa cell line, which was derived from African American Henrietta Lacks (38).
Between this early report and today, the problem has intensified (36). In “Human Cell Cross-Contamination Since 1983,” Masters reported results from a Medline search for the years 2000–2004 revealing 19 citations for the putative intestinal cell Int 407, 45 citations for the putative amnion cell WISH, 59 citations for the putative liver cell Chang liver, 470 citations for the putative human nasal carcinoma cell Hep-2, and 556 citations for the putative oral carcinoma KB (37). Masters reported hundreds of papers being published each year in high-impact journals using these cell lines, yet fewer than 10% of the papers reveal the model system being used is in fact HeLa cells (37).
Over 220 publications were found by Buehring et al. (23) in a PubMed database search from 1969 to 2004, in which known HeLa contaminants were used as a model for the tissue type of the original cell line (23). Furthermore, the authors collected survey data from mammalian cell biologists to determine how many were using HeLa contaminants without being aware of their true identity and how many were not using available means to ensure correct identity. The survey respondents included scientists, staff, and graduate students in 48 countries. The survey revealed of the 483 respondents, 32% used HeLa cells, 9% unwittingly were using HeLa contaminants, nearly half (46%) never tested for cell identity, 35% obtained all cell lines from another laboratory, and 63% obtained at least one cell line from another laboratory rather than from a major repository.
In 2005, Melcher et al. reported the putative normal colon epithelial cell line NCOL-1 (commonly used for colon cancer research) was not representative of a normal colon epithelial cell line. The researchers used spectral karyotyping to show this cell line is identical to LoVo, a cell line derived from a colon carcinoma (39). Yoshino et al. used short tandem repeat (STR) polymorphism analysis to examine approximately 400 cell lines in the Cell Engineering Division of the Japanese research institution RIKEN and found that 10 of the human cell lines were identical to a different cell line that had been earlier deposited in the collection and that had been misidentified by the depositor (40). Liscovitch et al. reported the misidentification of a human breast adenocarcinoma cell line, MCF7/AdrR, which has been used in over 300 molecular studies of cancer cell drug resistance. This cell line, originally thought to be from the parental MCF7 cell line, was recently matched to the OVCAR-8 ovarian adenocarcinoma (41), suggesting many studies will need further verification of results against the OVCAR-8 parental cell line. Azari et al. used a technique called combined DNA index system (CODIS), which uses tetrameric STR sequences on 13 distinct chromosomes to create a fingerprint that has a random match probability of one in a trillion (42). Using this technique, the authors examined 100 cell lines deposited in the National Cell Bank of Iran and compared them to STR reference profiles obtained from ATCC and the Japanese Collection of Research Bioresources cell bank. They found that 18.8% of the cell lines examined had been cross-contaminated (42).
Discussion
The number of publications containing spurious data as a result of over-passaged, misidentified, or contaminated cell lines is unknown. Drexler et al. summarizes, “the problem of false cell lines operates as a classic positive feedback loop, whereby information about false cell lines goes unreported or buried in specialist journals escaping the attention of unwary beginners who may later become victims or even perpetrators” (29).
The existing data and reports of wasted time and funds underscore the need for establishing proper controls and standards for cell culture conditions. Scientists have choices and can adopt safeguards. Researchers can perform authentication tests or, when possible, acquire cell cultures from reputable sources, such as nationally and internationally recognized cell banks where tested, identity-verified, contamination-free lines are distributed (23,32,43–45). Old cell stocks and absent records of source or passage number can be replaced with nominal investments, especially when compared with the costs of publishing misleading data. For those scientists working on cell lines derived themselves or received from a colleague, basic authentication tests such as STR profiling, isoenzyme analysis, and contamination tests are readily available and should be routinely used (11,12,28,32,46,47). Transferring cell lines to colleagues should be avoided, or when it does occur, accompanied with comprehensive documentation verifying the integrity of the material or tests need to be repeated (36).
The basis for any research, development, or production program involving cell cultures is the selection of an identity-verified and low-passage cell line. The use of similar and identified passage numbers throughout a project will better ensure reproducible results and comparisons between laboratories. To further ensure the use of authenticated cell lines, full cell line documentation, including the source and passage numbers used during experiments, should be submitted for scientific publications (28,31,34,36). Cell lines are critical components of experiments and should be considered as standard research reagents and given the same care and quality control measures that surround the use of kits, enzymes, and other laboratory products commercially obtained.
Until a greater consciousness and consensus regarding authentication compliance within the scientific community is achieved, malperforming and/or contaminated cell lines will continue to be released into the research community and spurious scientific conclusions will continue to affect the credibility and integrity of all biomedical science.
Acknowledgements
We thank Bettyanne Eilers, Aina Downing, and Scott Jenkins for their valuable assistance.
Competing Interests Statement
The authors declare no competing interests.
References
- 1. . 1997. Influence of culture time and passage number on morphological and physiological development of Caco-2 cells. Proc. Soc. Exp. Biol. Med 214:248–257.
- 2. . 1997. Effect of passage number on cellular response to DNA-damaging agents: cell survival and gene expression. Cancer Lett 113:77–86.
- 3. . 1997. LNCaP prostatic adenocarcinoma cells derived from low and high passage numbers display divergent responses not only to androgens but also to retinoids. J. Steroid Biochem. Mol. Biol 62:391–399.
- 4. . 1993. Effect of culture conditions on androgen sensitivity of the human prostate cancer cell line LNCaP. Prostate 23:213–223.
- 5. . 2005. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 21:1–26.
- 6. . 1997. Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages. Pharm. Res. 14:757–762.
- 7. . 2004. Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci. Rep. 24:631–639.
- 8. 2005. Wise Marketing Consultancy. Study Report: Cell Lines and Their Use in Research.
- 9. . 2003. Do cell culture conditions influence the carrier-mediated transport of peptides in Caco-2 cell monolayers? Eur. J. Pharm. Sci. 19:433–442.
- 10. . 1997. Identity of original and late passage Dami megakaryocytes with HEL erythroleukemia cells shown by combined cytogenetics and DNA fingerprinting. Leukemia 11:2032–2038.
- 11. . 1991. Active hexose transport across cultured human Caco-2 cells: characterization and influence of culture conditions. Biochim. Biophys. Acta. 1066:175–182.
- 12. . 2000. Interpretation of cell culture phenomena. Methods Cell Sci. 22:79–81.
- 13. . 1996. Transport properties are not altered across Caco-2 cells with heightened TEER despite underlying physiological and ultrastructural changes. J. Pharm. Sci. 85:270–273.
- 14. . 2003. Permeability characteristics of parental and clonal human intestinal Caco-2 cell lines differentiated in serum-supplemented and serum-free media. Toxicol. In Vitro 17:761–767.
- 15. . 1997. Analysis of drug permeation across Caco-2 monolayer: implication for predicting in vivo drug absorption. Pharm. Res. 14:486–491.
- 16. . 1991. The human prostatic cancer cell line LNCaP and its derived sublines: an in vitro model for the study of androgen sensitivity. J. Steroid Biochem. Mol. Biol. 40:207–214.
- 17. . 1984. A high-resolution study of chromosome changes in a human prostatic carcinoma cell line (LNCaP). Cancer Genet. Cytogenet. 11:399–404.
- 18. . 2004. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22:53–54.
- 19. . 2004. Comparative genomic hybridization and karyotyping of human embryonic stem cells reveals the occurrence of an isodicentric X chromosome after long-term cultivation. Mol. Hum. Reprod. 10:461–466.
- 20. . 2005. Preserving the genetic integrity of human stem cells. Nat. Biotechnol. 23:19–20.
- 21. . 2005. Genomic alterations in cultured human embryonic stem cells. Nat. Genet. 37:1099–1103.
- 22. . 2007. Passage number affects the pluripotency of mouse embryonic stem cells as judged by tetraploid embryo aggregation. Cell Tissue Res. 327:607–614.
- 23. . 2004. Cell line cross-contamination: how aware are mammalian cell culturists of the problem and how to monitor it? In Vitro Cell. Dev. Biol. Anim. 40:211–215.
- 24. . 1999. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int. J. Cancer 83:555–563.
- 25. . 2001. Cell banks detect false cell lines: journals must act too. Lancet Oncol. 2:467–468.
- 26. . 1952. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 12:264–265.
- 27. 1974. HeLa cells: contamination cultures around the world. Science 184:1058–1059.
- 28. . 1999. False human hematopoietic cell lines: cross-contaminations and misinterpretations. Leukemia 13:1601–1607.
- 29. . 2003. False leukemia-lymphoma cell lines: an update on over 500 cell lines. Leukemia 17:416–426.
- 30. . 2003. Persistent use of false myeloma cell lines. Hum. Cell 16:101–105.
- 31. . 1957. In R.W. Begg (Eds.), Canadian Cancer Conference. Vol. 2. Academic Press, New York.
- 32. . 1998. Cell cross-contamination in cell cultures: the silent and neglected danger. In Vitro Cell. Dev. Biol. Anim. 34:1–8.
- 33. . 1981. Cross-contamination of cells in culture. Science 212:446–452.
- 34. 2001. Cell culture forensics. Proc. Natl. Acad. Sci. USA 98:7656–7658.
- 35. . 1957. Transformation of normal cells in tissue culture: its significance relative to malignancy and virus vaccine production. Br. J. Exp. Pathol. 38:138–154 .
- 36. 2000. Cell contamination leads to inaccurate data: we must take action now. Nature 403:356.
- 37. 2004. Human cell cross-contamination since 1983. In Vitro Cell. Dev. Biol. Anim. 40:10.
- 38. 1968. Apparent HeLa cell contamination of human heteroploid cell lines. Nature 217:750–751.
- 39. . 2005. SKY and genetic fingerprinting reveal a cross-contamination of the putative normal colon epithelial cell line NCOL-1. Cancer Genet. Cytogenet. 151:84–87.
- 40. . 2006. Essential role of gene profiling analysis in the authentication of human cell lines. Hum. Cell 19:43–48.
- 41. . 2007. A case study in misidentification of cancer cell lines: MCF/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 245:350–352.
- 42. . 2007. Profiling and authentication of human cell lines using short tandem repeat (STR) loci: report from the national cell bank of Iran. Biologicals 35:195–202.
- 43. 2007. Cases of mistaken identity. Science 315:928–931.
- 44. . 2002. Good cell culture practice: ECVAM good cell culture practice task force report 1. Altern. Lab. Anim. 30:407–414.
- 45. 1992. DNA fingerprinting transforms the art of cell authentication. Nature 357:261–262.
- 46. . 2002. Comprehensive cytogenetic and molecular genetic characterization of TI-1 acute myeloid leukemia cell line reveals cross-contamination with K-562 cell line. Blood 99:1874–1876.
- 47. . 2005. Reconsidering cell line cross-contamination in NCOL-1. Cancer Genet. Cytogenet. 163:95–96.
- 48. 1973. Extrinsic cell contamination of tissue cultures, p. 1–27. In J. Fogh (Ed.), Contamination in Tissue Culture. Academic Press, New York.
- 49. . 1974. Banded marker chromosomes as indicators of intraspecies cellular contamination. Science 184:1093–1096.
- 50. . 1976. Identification of cells in culture. Am. J. Hematol. 1:237–242.
- 51. . 1977. Inter- and intraspecies contamination of human breast tumor cell lines HBC and BrCa5 and other cell cultures. Science 195:1343–1344.
- 52. . 1979. Mutually exclusive genetic signatures of human breast tumor cell lines with a common chromosomal marker. Cancer Res. 39:919–922.
- 53. . 1981. Contamination of Hodgkin's disease cell cultures. Nature 289:228–230.
- 54. . 1984. Cell characterization by use of multiple genetic markers, p. 13–31. In R.T. Acton and D.J. Lynn (Eds.), Eukaryotic Cell Cultures. Plenum Press, New York.
- 55. . 1988. Gene probes to detect cross-culture contamination in hormone producing cell lines. In Vitro Cell. Dev. Biol. 24:1971–1976.
- 56. . 1993. Multiparameter approach in the identification of cross-contaminated leukemia cell lines. Leuk. Lymphoma 10:359–368.
- 57. . 1995. Cell cross-contamination of U-937. J. Leukoc. Biol. 57:804.
- 58. . 2000. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab. Invest 80:37–45.












