Diagnosis of genitourinary tuberculosis by loop-mediated isothermal amplification based on SYBR Green I dye reaction
Abstract
A multitargeted loop-mediated isothermal amplification (MT-LAMP) assay targeting mpt64 (Rv1980c) and IS6110 was designed to diagnose genitourinary tuberculosis (GUTB) cases. While assessing gel-based, hydroxynaphthol blue (HNB) and SYBR Green I MT-LAMP assays on GUTB specimens (n = 28) in a pilot study, both gel-based/SYBR Green I assays exhibited better sensitivity than HNB LAMP. Since SYBR Green MT-LAMP is easier to perform compared with a gel-based assay, a higher number of GUTB specimens (n = 55) were evaluated by SYBR Green MT-LAMP, wherein 85.5% sensitivity and 94.4% specificity (n = 36) were obtained. Moreover, the sensitivity attained by MT-LAMP was significantly higher (p < 0.05) than with multiplex-PCR (mpt64 + IS6110). After further validating these MT-LAMP data in different epidemiological settings, this assay may be developed as a diagnostic kit.
METHOD SUMMARY
Multitargeted loop-mediated isothermal amplification (MT-LAMP) using gel-based and hydroxynaphthol blue (HNB)/SYBR Green I dye reactions were evaluated for the diagnosis of genitourinary tuberculosis, wherein both gel-based and SYBR Green I MT-LAMP exhibited a bit better diagnostic yield than HNB dye. The utility of SYBR Green MT-LAMP was further validated in a larger number of samples.
Graphical abstract
Graphical representation of gel-based/SYBR Green I multi-targeted LAMP (MT-LAMP) and multiplex-PCR (M-PCR) assays to diagnose genitourinary TB patients.

Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a major public health concern worldwide, ranking above HIV/AIDS. Until the COVID-19 pandemic, TB was the foremost cause of death from a single infectious agent and is now the second leading infectious killer after COVID-19 [1]. India harbors the highest TB burden (26%) globally followed by China (8.5%) and Indonesia (8.4%) [1]. Genitourinary TB (GUTB) is the second most common extrapulmonary TB (EPTB), accounting for 15–20% of EPTB [2]. Clinical manifestations of GUTB are nonspecific, causing infertility, kidney dysfunction, ureteral stricture and shrunken bladder [3]. A diagnostic dilemma arises due to the varied clinical presentations, mimicry of GUTB by urinary tract infection (UTI) of non-TB origin and low positivity by bacteriological (smear/culture) tests owing to low bacillary load in clinical specimens [4,5]. Concurrently, definite diagnosis is not possible with hysterosalpingogram/intravenous urography or by laparoscopy, necessitating culture/histopathology or nucleic acid amplification tests (NAATs) as confirmatory tests [6]. However, except for the presence of acid-fast bacilli (AFB), histopathological examination (HPE) is not pathognomonic as it cannot differentiate GUTB from the other granulomatous diseases (e.g., fungal infection, sarcoidosis, etc.) [7,8]. Moreover, NAATs, such as PCR/multiplex-PCR (M-PCR), real-time PCR and GeneXpert often yield false-positive or false-negative results and require expensive instruments [7,9].
Conversely, loop-mediated isothermal amplification (LAMP) assays can be performed with minimal resources without a thermocycler and have the potential to revolutionize TB diagnostics [10]. Besides read-out amplicons on gel, LAMP products can be visualized by using either fluorescent dyes, such as propidium iodide, pico green and SYBR Green I, or the nonfluorescent dyes, such as hydroxylnaphthol blue (HNB), calcein, malachite green and Leucocrystal Violet [10–13]. Moreover, visual detection of LAMP products eliminates the requirement for a gel documentation system [10,11], thus reducing the cost of the assay. LAMP assays have been developed using various target genes, such as IS6110, 16S rRNA DNA (rrs), mpt64 (Rv1980c), pstS1 (Rv0934), gyrB (Rv0005) and so on, for the diagnosis of pulmonary TB/EPTB with promising results [10,13,15], yet limited information is available to diagnose GUTB by LAMP [14,16]. Markedly, multitargeted LAMP (MT-LAMP) using two targets (e.g., mpt64 and IS6110 or mpt64 and pstS1) revealed better sensitivity than the single-targeted LAMP for diagnosing osteoarticular TB (OATB) and TB meningitis [10,13,17]. Therefore, this study was designed to assess an MT-LAMP assay using mpt64 and IS6110 for GUTB diagnosis, wherein gel-based LAMP was compared using visualization methods (i.e., HNB and SYBR Green I dye reactions). After establishing the utility of SYBR Green I MT-LAMP to diagnose GUTB in a pilot study, the SYBR Green I results were validated in a larger number of specimens and compared with M-PCR (mpt64 + IS6110).
Materials & methods
Samples collection & inclusion/exclusion criteria
A total of 55 clinical specimens from suspected GUTB patients, including 25 endometrial biopsies (EBs) and 30 early morning midstream urine specimens with symptoms of dysuria, pyuria, hematuria, recurrent UTI, infertility among men/women and menstrual irregularities in women were obtained from the outpatient department or were admitted to the hospital of Pt. B.D. Sharma University of Health Sciences (UHS), Rohtak, India. This prospective study was carried out from March 2019 to December 2021 and approved by the Institutional Human Ethics Committee (IHEC) of Maharshi Dayanand University (MDU), Rohtak, India (IHEC/19/02 and HEC/2021/306). Written informed consent was taken from all patients, who underwent routine laboratory/radiological investigations under the supervision of a urologist/gynecologist.
Patients were broadly categorized as: confirmed GUTB cases (n = 6) based on positive smear microscopy for AFB by Ziehl-Neelsen staining/culture-positive for Mtb identification on Lowenstein-Jensen (LJ) medium, positive HPE or GeneXpert [18,19]. The presence of typical caseating granulomas/Langhans cells by HPE or the presence of AFB within EBs indicated tubercular involvement [20]. Clinically suspected GUTB cases (n = 49) were all smear/culture-negative but strongly suspected on the basis of clinical features, Mantoux test, imaging, cytological observations, IS6110 PCR and response to antitubercular therapy (ATT). In addition, nontuberculosis controls (non-TB controls) (n = 36) were included that comprised patients suffering from UTI of non-TB origin (n = 15), renal stone (n = 12) and women with menstrual irregularities of non-TB origin (n = 9). Exclusion criteria included patients already on ATT or not responding to ATT after 8 weeks, individuals with pulmonary TB and other EPTB types but not of GUTB, HIV-coinfected individuals and proven multidrug-resistant/extensively-drug resistant TB at the time of sample collection. Individuals suffering from diabetes mellitus or other comorbidities, like cancer, patients with psychiatric dysfunctions/autoimmune disorders and pregnant women were also excluded [18,19]. All patients were aged 21 to 55 years, comprising 38.5% males and 61.5% females.
In a pilot study, 44 patients were randomly chosen (28 clinically suspected GUTB cases and 16 non-TB controls) and were subjected to gel-based, HNB and SYBR Green I MT-LAMP assays (Figure 1A). Further, the SYBR Green I MT-LAMP assay was authenticated with a larger sample size (n = 91) as detailed in Figure 1B for categorization of GUTB specimens/non-TB controls and results were compared with M-PCR results.

(A) The flowchart for grouping of study participants evaluated by gel-based, SYBR Green I and HNB MT-LAMP and (B) the flowchart for grouping of the study participants for evaluating SYBR Green I MT-LAMP and M-PCR assays.
HNB: Hydroxynaphthol blue; M-PCR: Multiplex-PCR; MT-LAMP: Multitargeted loop-mediated isothermal amplification.
Processing of genitourinary tuberculosis specimens & DNA extraction
First, EB specimens were cut into small pieces using a sterile scalpel followed by grinding with a glass tissue homogenizer [13]. Urine/EB homogenates were processed using 1% N-acetyl L-cysteine+4% NaOH in a biosafety hood and neutralized with phosphate-buffered saline followed by incubation at 37°C for 20 min and centrifugation at 3000X g for 15 min. Pellets obtained from EBs and urine were subjected to DNA extraction using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) and QIAamp DNA blood mini kit, respectively. Aliquots of the sediments were taken for smear microscopy and inoculated on LJ medium for culture.
Primers for loop-mediated isothermal amplification/multiplex-PCR assays
For LAMP, primers for Mtb IS6110, mpt64 and pstS1 targets were utilized as previously detailed [13], while primers for fbpB (Rv1886c) were designed using software version 1.15 (Optigene, Horsham, UK, Table 1). The LAMP required a set of six primers comprising two outer primers (forward outer primer [FOP] and backward outer primer [BOP]), two inner primers (forward inner primer [FIP] and backward inner primer [BIP]) and two loop primers (forward loop primer [FLP] and backward loop primer [BLP]). For M-PCR, primers for IS6110 were employed as previously described [21], while primers for mpt64 were manually designed. Primers were purchased from Eurofins Genomics India Pvt. Ltd., Bangaluru, India.
| Assay | Gene target | Primer name | Primer sequences (5′-3′) | Primer length (bp) |
|---|---|---|---|---|
| LAMP | mpt64 | FOP | CCCCCGGGTTGAAGAAGA | 18 |
| BOP | GGCCTATCGCAAGCCAAT | 18 | ||
| FIP | GTATCGATAGCGCCGAATGCCGTTTTCGTTCGTGACTGCGAAGT | 44 | ||
| BIP | TGCTTGCTCAGTTCACCTTGCATTTTCACCTATGACACGCTGTGG | 45 | ||
| FLP | GCTTGGACCCGGTGAATTATCAGA | 24 | ||
| BLP | AGCGGATCGGTGTCAGCCT | 19 | ||
| IS6110 | FOP | CTCGACATCCTCGATGGA | 18 | |
| BOP | GATCGAGCAAGCCATCTG | 18 | ||
| FIP | AGCGGTCGGAAGCTCCTAGGTCTTGTATAGGCCGTTG | 37 | ||
| BIP | ACCGGATCGATGTGTACTGAGAAAGCGTACTCGACCTGAA | 40 | ||
| FLP | AATGCACTAGCCGAGACG | 18 | ||
| BLP | CCTATCCGTATGGTGGATAACG | 22 | ||
| pstS1 | FOP | CCAGGCGATTTCGATGAT | 18 | |
| BOP | CTAGCTGGAAATCGTCGC | 18 | ||
| FIP | ATGCCTGCAAGGTCTGCGCCGATCATCAACTACGAGTAC | 39 | ||
| BIP | TCACCGACGGCAACAAGGCAGACAACTTCACCACCG | 36 | ||
| FLP | TTGCCGGTTGTTGACGAT | 18 | ||
| BLP | GTTCCTCGACCAGGTTCATT | 20 | ||
| fbpB | FOP | ACATCAAGGTTCAGTTCCAG | 20 | |
| BOP | CCATCGACAAGCCGATTG | 18 | ||
| FIP | CGGCATGACTATCGACAGTCCTACAACGGCTGGGATATCA | 40 | ||
| BIP | CTTCTACAGCGACTGGTACAGCGCTGGTCAGGAAGGTTTC | 40 | ||
| FLP | GGTAGTACCACTCGAACGC | 19 | ||
| BLP | GGCTGCCAGACTTACAAGT | 19 | ||
| M-PCR | mpt64 | Forward | ACATCAGCCTGCCCAGTTACTAC | 23 |
| Reverse | ATCGGTGTCAGCCTGCCAC | 19 | ||
| IS6110 | Forward | GGACAACGCCGAATTGCGAAGGGC | 24 | |
| Reverse | TAGGCGTCGGTGACAAAGGCCACG | 24 |
Evaluation of clinical genitourinary tuberculosis specimens by loop-mediated isothermal amplification & multitargeted loop-mediated isothermal amplification assays
The analytical sensitivity of the purified DNA was determined by preparing its serial tenfold dilutions (from 104 fg/μl to 0.1 fg/μl) and analyzed by LAMP (both gel-based and HNB/SYBR Green I dye reaction). In a preliminary study, gel-based LAMP was performed [13] on a few clinical specimens (n = 12) using a set of three primer pairs each for the mpt64, IS6110, pstS1 and fbpB genes to choose the two best targets (mpt64 and IS6110) for MT-LAMP. LAMP was carried out in a total volume of 25 μl comprising 2.5 μl 10X Bst polymerase buffer, 1.2 mM each of dNTPs, 6 mM MgSO4, 0.2 mM each of FOP and BOP, 1.6 mM each of FIP and BIP, 0.8 mM each of FLP and BLP, 0.8 M betaine, 320 U/ml of Bst DNA polymerase, 1 μl of template DNA (extracted from clinical specimens) and PCR-grade water. LAMP reaction was carried out in a dry water bath at a constant temperature of 65°C for 60 min, which was terminated by heat inactivation at 80°C for 5 min. After amplification, LAMP products were resolved by 2% agarose gel electrophoresis when viewed under the gel documentation system. Both positive (purified Mtb H37Rv DNA, 1 ng/ml) and negative (no template DNA) controls were also run. MT-LAMP was considered positive when found positive either by mpt64 LAMP or IS6110 LAMP or by both.
Additionally, LAMP products were detected by a color change when 1 μl of SYBR Green I dye solution (1:10 dilution) was added to the reaction mixture postamplification followed by incubation in the dark for 5 min [22]. A positive test showed a color change from orange to green fluorescence when viewed under a UV transilluminator. However, 1 μl of 3 mM HNB dye solution was added to the reaction mixture prior to amplification, wherein a positive test showed a color change from violet to sky blue in visible light [13]. Both positive (purified Mtb H37Rv DNA, 10 fg/μl) and negative (no template DNA) controls were included. All clinical specimens were run in duplicate to ensure the reproducibility of results in all LAMP assays.
Evaluation of clinical samples by multiplex-PCR
For M-PCR, the method described by Raj et al. [23] was followed with some modifications: 2–5 μl of DNA (extracted from clinical specimens) was added to 25 μl of the PCR master mix comprising 2.5 μl 10X PCR buffer, 1.5 μl of 2.5 mM each of dNTPs, 1 μl of 25 mM MgCl2, 10 pM of each forward and reverse primers for mpt64 and IS6110 in a ratio of 0.2:0.2 μM, 1 U of Taq DNA polymerase and PCR-grade water. The specific bands of 984 bp and 300 bp were observed for IS6110 and mpt64, respectively. The presence of either a single band or both bands confirmed the presence of Mtb DNA in clinical specimens. Both positive (purified Mtb H37Rv DNA, 1 ng/ml) and negative (no template DNA) controls were run.
Statistical analyses
Sensitivities and specificities of various MT-LAMP assays and M-PCR were determined at 95% CIs against the composite reference standard (CRS; a combination of clinical features, smear/culture, imaging, HPE, cytological observations, IS6110 PCR, GeneXpert, response to ATT, etc.) [24]. Moreover, a comparison was made between the SYBR Green I MT-LAMP and M-PCR using the exact symmetry test [13]. The statistical software STATA/SE version 14.2 (StataCorp LP, TX, USA) was used for the analysis. A value of p < 0.05 was considered statistically significant.
Results & discussion
A preliminary study demonstrated that among 12 clinically suspected GUTB cases, 10, 9, 8 and 8 cases were found to be positive by gel-based LAMP targeting mpt64, IS6110, pstS1 and fbpB, respectively (data not shown); sensitivity was in descending order as follows: mpt64>IS6110>pstS1/fbpB. Since the multiplexing approach to designing LAMP is tedious owing to the use of a higher number of primers per target to enhance the likelihood of primer–primer interactions [24], the two best targets from this study were chosen (mpt64 and IS6110) to develop an MT-LAMP.
Analytical sensitivity of purified Mycobacterium tuberculosis DNA by loop-mediated isothermal amplification assay
An LOD of 10 fg was determined for the purified Mtb DNA by gel-based mpt64/IS6110 LAMP (Figure 2A & B), which showed a ladder-like pattern(s). Both mpt64 SYBR Green I and HNB LAMP also revealed an LOD of 10 fg (Figure 3A & B). Likewise, an LOD of 10 fg was attained for IS6110 LAMP using SYBR Green I/HNB reactions (data not shown). These findings are in agreement with the previous report, wherein mpt64/IS6110/pstS1 gel-based/HNB LAMP showed an LOD of 10 fg for the purified Mtb DNA [13]. However, Aryan et al. [25] reported an LOD of 5 fg for the turbidity reaction, SYBR Green I and gel-based IS6110 LAMP. A similar LOD of 5 fg (corresponding to 1–1.3 copies of Mtb genome) was attained by Joon et al. [26], using sdaA (Rv0069c) LAMP combined with lateral flow dipstick (LFD) as well as sdaA SYBR Green I LAMP assay. Further, the results are consistent with the mpt64 SYBR Green I/HNB LAMP and TaqMan real-time PCR results with an LOD of 10 fg for the purified Mtb DNA [27,28]. Conversely, higher LODs of 50 fg, 100 fg, 1 pg and 5 pg were documented for dry methyl green-based rrs LAMP, IS6110 HNB/turbidity-based LAMP, calcein LAMP and IS6110 LAMP combined with LFD, respectively [11,12,29].

(A)mpt64 LAMP and (B) IS6110 LAMP. For both the assays, Lane M, molecular marker (100 bp DNA ladder); Lanes 1–6, serial tenfold dilutions of purified DNA (ranging from 104 fg/μl to 0.1 fg/μl); Lane 7, negative control (no template DNA).
LAMP: Loop-mediated isothermal amplification.
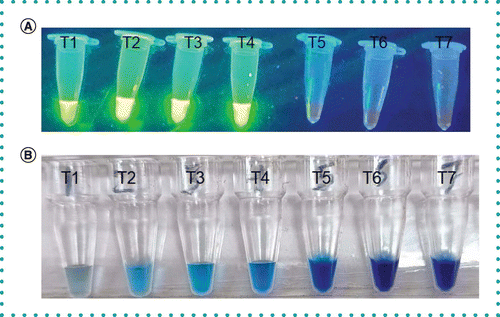
(A) Visualization of purified Mycobacterium tuberculosis H37Rv DNA by mpt64 SYBR Green I LAMP reaction: serial tenfold dilutions of purified DNA (ranging from 104 fg/μl to 0.1 fg/μl) added in tubes T1–T6 showed green fluorescence in tubes T1–T4 using UV transilluminator; Tubes T5 and T6, negative results; Tube T7, negative control (no template DNA), and (B) visualization of purified Mycobacterium tuberculosis H37Rv DNA by mpt64 HNB LAMP reaction: serial tenfold dilutions of purified DNA (ranging from 104 fg/μl to 0.1 fg/μl) added in tubes T1–T6 showed a color change from violet to sky blue in tubes T1–T4; Tubes T5 and T6, negative results; Tube T7, negative control (no template DNA).
LAMP: Loop-mediated isothermal amplification.
Pilot study: gel-based versus hydroxylnaphthol blue/SYBR Green I multitargeted loop-mediated isothermal amplification in clinically suspected genitourinary tuberculosis
A sensitivity of 82.1% (95% CI: 63.1–93.9%) was obtained in clinically suspected GUTB (n = 28) cases by gel-based MT-LAMP against CRS, which also matched the SYBR Green I assay (Table 2). Concurrently, the HNB MT-LAMP exhibited lesser sensitivity of 75% (95% CI: 55.1–89.3%), wherein two GUTB specimens were found to be ambiguous since the color change was undetectable and results were interpreted as ‘indeterminate’, yet those specimens exhibited a clear-cut positive reaction by both SYBR Green I/gel-based MT-LAMP. Among 16 non-TB controls, one and two false-positive cases were noted with gel-based/SYBR Green I and HNB MT-LAMP, leading to specificities of 93.7% (95% CI: 69.7–99.8%) and 87.5% (95% CI: 61.7–98.5%), respectively. Markedly, both gel-based and SYBR Green I MT-LAMP revealed similar sensitivity (82.1%) and specificity (93.7%), which is in agreement with previous reports [10,30] for diagnosing pulmonary TB and OATB by mpt64 LAMP and MT-LAMP (using mpt64 and IS6110), respectively, wherein the respiratory/OATB specimens positive by gel-based mpt64 LAMP/MT-LAMP were also positive by SYBR Green I. Likewise, Chen et al. [31] demonstrated no discrepancy among gel-based and SYBR Green LAMPs, while the brightness of HNB dye was significantly weaker than SYBR Green [31], as observed in the current study. Since SYBR Green MT-LAMP is comparatively easier to perform and more rapid than the gel-based assay, this assay was corroborated in a larger number of specimens.
| MT-LAMP assay used | Clinically suspected GUTB cases (n = 28) | Non-TB controls (n = 16) | ||
|---|---|---|---|---|
| (+) | Sensitivity (%) | (+) | Specificity (%) | |
| Gel based | 23 | 82.1 (63.1–93.9) | 1 | 93.7 (69.7–99.8) |
| HNB | 21 | 75 (55.1–89.3) | 2 | 87.5 (61.7–98.5) |
| SYBR Green I | 23 | 82.1 (63.1–93.9) | 1 | 93.7 (69.7–99.8) |
Turbidity measurement is considered the simplest method to monitor LAMP products, but at times it is not feasible for visualizing a tiny white pellet (collected following a short spin) by the naked eye or quantifying the same by a real-time turbidity meter, which may lead to erroneous results [32,33]. Moreover, turbidity persists only for a short duration, which usually causes a judgment error [33]. Identification of isothermally amplified DNA by SYBR Green, rather, seems to be a useful approach that emits a weak fluorescence signal in presence of single-stranded DNA but emits strongly upon binding to double-stranded DNA [32]. However, SYBR Green is often associated with aerosol contamination, since it is added postamplification [32], which may be reduced by following strict laboratory practices, using the dUTP-uracil-N-glycosylase (dUTP-UNG) approach or exploiting LAMP combined with LFD assay [12,26,34,35].
Comparison of SYBR Green I multitargeted loop-mediated isothermal amplification versus multiplex-PCR in genitourinary tuberculosis
The SYBR Green I MT-LAMP results were validated with a larger sample size (n = 91) and those results were compared with M-PCR results. Remarkably, all six confirmed GUTB cases were positive by SYBR Green I MT-LAMP, with a sensitivity of 100% (95% CI: 54.1–100%) against CRS (Table 3), whereas 83.7% (95% CI: 70.3–92.7%) sensitivity was attained by MT-LAMP in clinically suspected GUTB cases (n = 49). Further, M-PCR documented a significantly lower sensitivity (p < 0.05) than MT-LAMP in 55 total GUTB cases. SYBR Green MT-LAMP showed two false-positive results in 36 non-TB controls, resulting in 94.4% (95% CI: 81.3–99.3%) specificity, which could be due to carryover contamination from adding SYBR Green postamplification [32]. However, compared with other studies [36,37], such an effect was less pronounced in this study as a result of following stringent laboratory practices [34], that is, preparing reaction mixtures for LAMP in a separate laminar flow placed in a different room that was apart from the dry water bath and the post-LAMP processing area (performed in a separate laminar flow) and immediately disposing of the biohazard materials after completing the experiment. Moreover, negative/positive controls were run in all LAMP assays. Concurrently, cross-contamination could be prevented by adding dUTP-UNG in a LAMP reaction, wherein dTTP is replaced with dUTP [16,35]. The combined use of sdaA LAMP and LFD has also been documented [26], wherein a labeled probe was employed as FLP/BLP for the simultaneous amplification and hybridization that reduced cross-contamination with no adverse effect on amplification efficiency. Likewise, Kaewphinit et al. [12] developed an IS6110 LAMP combined with LFD to diagnose pulmonary TB that showed a high diagnostic yield against culture. Additionally, the use of dry methyl green dye-based LAMP has been reported to avoid cross-contamination within sputa of pulmonary TB [11] with good sensitivity (92.8%) and specificity (96.3%) in culture-positive and culture-negative cases, respectively [11]. However, one needs to be cautious to interpret specificity in culture-negative cases, since a number of paucibacillary pulmonary TB specimens are culture-negative but are considered clinically suspected TB cases based on clinical features, radiological findings and response to ATT [38].
| Category of GUTB | MT-LAMP | M-PCR | ||||
|---|---|---|---|---|---|---|
| (+) | Sensitivity (%) | Specificity (%) | (+) | Sensitivity (%) | Specificity (%) | |
| Confirmed (n = 6) | 6 | 100 (54.1–100) | 5 | 83.3 (35.9–99.6) | ||
| Clinically suspected (n = 49) | 41 | 83.7 (70.3–92.7) | 33 | 67.4 (52.5–80.1) | ||
| Total GUTB (n = 55) | 47 | †85.5 (73.3–93.5) | 38 | †69.1 (55.2–80.9) | ||
| Non-TB controls (n = 36) | 2 | 94.4 (81.3–99.3) | 4 | 88.9 (73.9–96.9) | ||
Representative examples of GUTB specimens by SYBR Green MT-LAMP and M-PCR are shown in Figures 4 and 5, respectively. Markedly, a κ-value of 0.252 was noted between the SYBR Green MT-LAMP and M-PCR with a fair agreement (p < 0.05) between the two assays. Among 55 total GUTB cases analyzed by SYBR Green MT-LAMP, four cases were picked by mpt64 LAMP alone and one was picked by IS6110 LAMP alone, thus providing an additional detection benefit of 9.1% (5/55) cases. Overall, 85.5% sensitivity and 94.4% specificity were obtained by SYBR Green MT-LAMP in total GUTB cases, while lesser sensitivities of 69.1% and 76.4% were attained with single-targeted IS6110 LAMP and mpt64 LAMP, respectively (data not shown). Furthermore, the IS6110 LAMP results are in agreement with the previous report on female genital TB diagnosis within EBs [14], where 66.2% sensitivity and 92.7% specificity were obtained.

Tube T1, positive control (purified Mycobacterium tuberculosis DNA, 1 pg/μl); Tube T2, negative control (no template DNA); Tubes T3, T4 and T8, positive genitourinary tuberculosis specimens and Tubes T5–T7, nontuberculosis controls.

Lane M, molecular marker (100 bp DNA ladder); Lane 1 and 14, positive control (purified Mycobacterium tuberculosis DNA, 10 ng/ml) showed two bands each of 984 bp and 300 bp for IS6110 and mpt64, respectively; Lane 2, negative control (no template DNA); Lane 3, 4, 9, 11, nontuberculosis controls; Lane 13, negative control (only PCR-grade water); Lane 5–8, 10, 12, positive genitourinary tuberculosis specimens.
Although IS6110 is the most favorable target for NAATs including LAMP, being a repetitive target sequence, the lesser sensitivity achieved with IS6110 LAMP in this study could be due to zero or low copy numbers of IS6110 in 10–40% of Indian clinical isolates [13,23], thus the inclusion of mpt64 and IS6110 in MT-LAMP could enhance the true-positive outcomes (Table 3). The SYBR Green MT-LAMP results are almost in concurrence with previous findings on TB meningitis and OATB diagnoses, where sensitivities of 84–88% and specificities of 94–100% were acquired by gel-based/SYBR Green MT-LAMP using mpt64 and IS6110 or mpt64 and pstS1 targets [13,17]. Furthermore, Joon et al. [16] demonstrated high sensitivity (92.5%) and specificity (99.2%) by sdaA SYBR Green LAMP in EPTB specimens including GUTB against CRS (comprised of culture and IS6110/mpt64 PCR). High specificity attained in their study [16] could be due to the addition of dUTP-UNG in a LAMP reaction that might eliminate carryover contamination, resulting in diminished false-positive results. However, other important parameters (i.e., imaging, HPE, cytology and GeneXpert) were not included in the CRS of their study [16] as recommended by the index TB guidelines on EPTB including GUTB [39]. Of note, both culture and IS6110/mpt64 PCR themselves yield low to moderate sensitivities in most EPTB specimens including GUTB [7–9], thus their use as CRS could lead to overestimated sensitivity of the tested diagnostic method [40,41].
Subsequently, moderate sensitivity of 69.1% and 88.9% specificity were obtained by M-PCR, though lesser sensitivity (43%) and specificity (81.3%) were acquired in menstrual blood/EBs of female genital TB cases by M-PCR using the same targets [42]. The different sensitivities attained in the two studies could be due to the different nature of GUTB specimens. Overall, we achieved good sensitivity (82.1–85.5%) and specificity (∼94%) to diagnose GUTB by SYBR Green MT-LAMP against CRS, which matches the sensitivity of WHO [43] guidelines (sensitivity of ≥80% and specificity of ≥98% for EPTB including GUTB) to develop new diagnostic tests. However, the specificity of the assay needs to be further improved, which is rather more important than sensitivity in low-endemic countries with a low incidence of TB including GUTB so as to secure an appropriate positive predictive value [44,45]. This may be improved either by dUTP-UNG treatment or combined use of MT-LAMP with LFD strategy [12,16,26,35]. Moreover, Kim et al. [46] documented high sensitivity (100%) and specificity (100%) within sputa and bronchial washings of pulmonary TB cases by real-time multiplex-LAMP targeting IS6110 + rpoB (Rv0664) against real-time PCR, which could detect 104 copies/μl of IS6110 plasmid and also differentiate Mtb and nontubercular mycobacteria. Additionally, high sensitivity/specificity of 100% was obtained in EPTB specimens (GUTB specimens were not included) by real-time multiplex-LAMP [46], though the sample size was small (n = 17). Comparatively, SYBR Green MT-LAMP displays a bit lesser diagnostic accuracy in GUTB specimens, yet it is a quicker and low-cost method that does not require expensive real-time PCR equipment and skilled technicians [46].
Conclusion & future perspective
The authors evaluated the gel-based and visualization methods (i.e., HNB and SYBR Green) to diagnose GUTB by MT-LAMP (mpt64 and IS6110) in a pilot study where gel-based/SYBR Green MT-LAMP exhibited better diagnostic yield than HNB MT-LAMP. Since SYBR Green MT-LAMP is relatively easier to perform than the gel-based assay, the SYBR Green MT-LAMP results were authenticated in a larger number of GUTB cases. To the best of our knowledge, this is the first report to detect paucibacillary GUTB specimens by SYBR Green MT-LAMP with encouraging results. The authors are currently focused on eliminating carryover contamination in the assay by dUTP-UNG treatment or combined use of MT-LAMP with LFD, as a result of which the specificity may be further improved. After validating these results in diverse geographical locations and reducing the cost (e.g., lesser duration of LAMP amplification, minimal usage of reagents, etc.), this MT-LAMP can be developed into an attractive kit for GUTB diagnosis and may later translate into a point-of-care test that can be utilized to diagnose other EPTB types.
Diagnosis of genitourinary tuberculosis (GUTB) is challenging owing to atypical clinical presentations and low bacillary load in clinical specimens.
The analytical sensitivity of purified Mycobacterium tuberculosis DNA was found to be 10 fg by mpt64 loop-mediated isothermal amplification (LAMP) and IS6110 LAMP for gel-based and hydroxynaphthol blue (HNB)/SYBR Green I dye reactions.
In a pilot study, 28 GUTB specimens and 16 non-TB controls were assessed by gel-based and visualization methods, including HNB/SYBR Green multitargeted (MT)-LAMP (using mpt64 and IS6110), wherein both SYBR Green/gel-based MT-LAMP revealed better sensitivity (82.1 vs 75%) and specificity (93.7 vs 87.5%) than HNB MT-LAMP.
Since SYBR Green MT-LAMP is comparatively easier and more rapid than the gel-based assay, the SYBR Green MT-LAMP results were replicated in a larger number of samples, with sensitivities of 83.7% and 85.5% obtained in clinically suspected (n = 49) and total GUTB (n = 55) cases, respectively, with 94.4% specificity (n = 36).
The sensitivity obtained by SYBR Green MT-LAMP was significantly higher (p < 0.05) than that obtained by M-PCR in total GUTB cases.
The specificity of SYBR Green MT-LAMP can be improved by dUTP-Uracil-DNA glycosylase treatment or combined use of MT-LAMP with lateral flow dipstick assay.
After further validating these results in diverse epidemiological settings, this MT-LAMP may be developed into a diagnostic kit and translated into a point-of-care test.
Author contributions
PK Mehta conceptualized and designed this study. E Kamra and A Khan were responsible for the collection/processing of clinical samples. E Kamra, N Singh, A Khan and J Singh carried out multitargeted loop-mediated isothermal amplification/multiplex-PCR assays. M Chauhan and H Kamal provided clinical samples and assisted in the interpretation of clinical data. PK Mehta and E Kamra wrote this manuscript and other coauthors edited the manuscript.
Acknowledgments
We are thankful to Mandira Verma, Vallabhbhai Patel Chest Institute, the University of Delhi for providing us with the purified Mycobacterium tuberculosis H37Rv DNA.
Financial & competing interests disclosure
PK Mehta acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi for awarding the Emeritus Scientist Fellowship (ES scheme no. 21(1068)/18/EMR-II) to pursue this study. E Kamra also acknowledges CSIR, New Delhi for awarding the Junior/Senior Research Fellowship. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This prospective study was carried out from March 2019 to December 2021 and approved by the Institutional Human Ethics Committee (IHEC) of MDU, Rohtak (IHEC/19/02 and HEC/2021/306). The written informed consent was taken from all the patients, who underwent routine laboratory/radiological investigations under the supervision of a urologist/gynecologist.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. WHO. Global Tuberculosis Report (2021). www.who.int/publications/i/item/9789240037021
- 2. . Urogenital tuberculosis – epidemiology, pathogenesis and clinical features. Nat. Rev. Urol. 16(10), 573–598 (2019). • Details the epidemiology, mode of transmission, clinical features and various modalities utilized for genitourinary tuberculosis diagnosis.
- 3. . Urinary polymerase chain reaction for diagnosis of urogenital tuberculosis. J. Urol. 5, 46–49 (2008).
- 4. . Urogenital tuberculosis, the cause of ineffective antibacterial therapy for urinary tract infections. Ther. Adv. Urol. 10(3), 95–101 (2018).
- 5. Utility of T-cell interferon-γ release assays for the diagnosis of female genital tuberculosis in a tertiary referral hospital in Beijing, China. Medicine (Baltimore) 95(44), e5200 (2016).
- 6. . Multiplex PCR from menstrual blood: a non-invasive cost-effective approach to reduce diagnostic dilemma for genital tuberculosis. Mol. Diagn. Ther. 22(3), 391–396 (2018).
- 7. . Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol. Med. Microbiol. 66(1), 20–36 (2012).
- 8. . Insight into diagnosis of female genital tuberculosis. Expert Rev. Mol. Diagn. 10, 1–18 (2022).
- 9. . Current updates in diagnosis of male urogenital tuberculosis. Expert Rev. Anti Infect. Ther. 19(10), 1175–1190 (2021).
- 10. . Diagnostic potential of multi-targeted LAMP (loop-mediated isothermal amplification) for osteoarticular tuberculosis. J. Orthop. Res. 35(2), 361–365 (2017).
- 11. Direct detection of Mycobacterium tuberculosis in clinical samples by a dry methyl green loop-mediated isothermal amplification (LAMP) method. Tuberculosis (Edinb.) 117, 1–6 (2019).
- 12. . Detection of Mycobacterium tuberculosis by using loop-mediated isothermal amplification combined with a lateral flow dipstick in clinical samples. Biomed. Res. Int. 2013, 926230 (2013).
- 13. Diagnosis of osteoarticular tuberculosis: multi-targeted loop-mediated isothermal amplification assay versus multiplex PCR. Future Microbiol. 16(13), 935–948 (2021). •• Describes the usefulness of gel-based and hydroxynaphthol blue/calcein loop-mediated isothermal amplification to diagnose osteoarticular tuberculosis.
- 14. . Loop-mediated isothermal amplification assay for detection of Mycobacterium tuberculosis complex in infertile women. Indian J. Med. Microbiol. 34(3), 322–327 (2016). • Demonstrates the use of IS6110 loop-mediated isothermal amplification to diagnose female genital tuberculosis.
- 15. . Diagnostic accuracy of the loop-mediated isothermal amplification assay for extrapulmonary tuberculosis: a meta-analysis. PLoS One 13(6), e0199290 (2018). •• Details the meta-analysis of loop-mediated isothermal amplification assays for extrapulmonary tuberculosis specimens.
- 16. . Loop-mediated isothermal amplification as alternative to PCR for the diagnosis of extra-pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 19(8), 986–991 (2015). • Describes the use of dUTP-Uracil-DNA glycosylase treatment in SYBR Green loop-mediated isothermal amplification reaction to avoid cross-contamination.
- 17. Multitargeted loop-mediated isothermal amplification for rapid diagnosis of tuberculous meningitis. Int. J. Tuberc. Lung Dis. 20, 625–630 (2016).
- 18. . Use of endo-ovarian tissue biopsy and pelvic aspirated fluid for the diagnosis of female genital tuberculosis by conventional versus molecular methods. PLoS One 9(5), e98005 (2014). •• Meticulously details the inclusion/exclusion criteria to select female genitourinary tuberculosis cases.
- 19. Chronic kidney disease with genitourinary tuberculosis: old disease but ongoing complication. BMC Nephrol. 19(1), 193 (2018).
- 20. . Efficacy and role of Xpert® Mycobacterium tuberculosis/rifampicin assay in urinary tuberculosis. Indian J. Urol. 34(4), 268–272 (2018).
- 21. . Differentiation of Mycobacterium tuberculosis complex from non-tubercular mycobacteria by nested multiplex PCR targeting IS6110, MTP40 and 32kD alpha antigen encoding gene fragments. BMC Infect. Dis. 16, 123 (2016).
- 22. . The development of a loop-mediated isothermal amplification method (LAMP) for Echinococcus granulosis coprodetection. Am. J. Trop. Med. Hyg. 87(5), 883–887 (2012).
- 23. Comparative evaluation of several gene targets for designing a multiplex-PCR for an early diagnosis of extrapulmonary tuberculosis. Yonsei Med. J. 57, 88–96 (2016).
- 24. Comparative evaluation of GeneXpert MTB/RIF and multiplex PCR targeting mpb64 and IS6110 for the diagnosis of pleural TB. Future Microbiol. 13(4), 407–413 (2018).
- 25. A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol. Res. 165, 211–220 (2010).
- 26. . Development and evaluation of rapid and specific sdaA LAMP-LFD assay with Xpert MTB/RIF assay for diagnosis of tuberculosis. J. Microbiol. Methods 159, 161–166 (2019). •• Describes the role of sdaA loop-mediated isothermal amplification combined with lateral flow dipstick assay to avoid cross-contamination.
- 27. . Development of a loop-mediated isothermal amplification assay targeting the mpb64 gene for diagnosis of intraocular tuberculosis. J. Clin. Microbiol. 51(11), 3839–3840 (2013).
- 28. . Loop-mediated isothermal amplification assay using hydroxynaphthol blue, conventional polymerase chain reaction and real-time PCR in the diagnosis of intraocular tuberculosis. Indian J. Med. Microbiol. 33(4), 568–571 (2015).
- 29. . Visual detection of Mycobacterium tuberculosis by loop-mediated isothermal amplification. Eur. Respir. J. 48, OA1509 (2016).
- 30. . A loop-mediated isothermal amplification assay for the diagnosis of pulmonary tuberculosis. Lett. Appl. Microbiol. 68(3), 219–225 (2019).
- 31. Endpoint visual detection of three genetically modified rice events by loop-mediated isothermal amplification. Int. J. Mol. Sci. 13(11), 14421–14433 (2012).
- 32. . Shining a light on LAMP assays' A comparison of LAMP visualization methods including the novel use of berberine. BioTechniques 58(4), 189–194 (2015). • Meticulously details various direct/indirect visualization methods to evaluate loop-mediated isothermal amplification products.
- 33. . Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J. Appl. Microbiol. 124(3), 626–643 (2018).
- 34. . Evaluation of two loop-mediated isothermal amplification methods for the detection of Salmonella enteritidis and Listeria monocytogenes in artificially contaminated ready-to-eat fresh produce. Ital. J. Food Saf. 4(3), 5383 (2015).
- 35. . Development and clinical evaluation of sdaA loop-mediated isothermal amplification assay for detection of Mycobacterium tuberculosis with an approach to prevent carryover contamination. J. Clin. Microbiol. 52, 2662–2664 (2014).
- 36. Loop-mediated isothermal amplification for rapid and reliable diagnosis of tuberculous meningitis. J. Clin. Microbiol. 49(5), 1861–1865 (2011).
- 37. . The application of IS6110-baced loop-mediated isothermal amplification (LAMP) in the early diagnosis of tuberculous meningitis. Oncotarget. 8(34), 57537–57542 (2017).
- 38. Serodiagnostic potential of immuno-PCR using a cocktail of mycobacterial antigen 85B, ESAT-6 and cord factor in tuberculosis patients. J. Microbiol. Methods 120, 56–64 (2016).
- 39. Ministry of Health and Family Welfare, Government of India and World Health Organization. INDEX TB guidelines: guidelines on extra-pulmonary tuberculosis for India. MoHFW, New Delhi, India (2016).
- 40. . Light-emitting diode fluorescence microscopy for tuberculosis diagnosis: a meta-analysis. Eur. Respir. J. 47(3), 929–937 (2016).
- 41. . Recent updates in diagnosis of abdominal tuberculosis with emphasis on nucleic acid amplification tests. Expert Rev. Gastroenterol. Hepatol. 16(1), 33–49 (2022).
- 42. . Quick diagnosis of female genital tuberculosis using multiplex fast polymerase chain reaction in Southern Assam, India. Int. J. Gynaecol. Obstet. 118(1), 72–73 (2012).
- 43. World Health Organization. High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting, 28–29 April 2014. WHO, Geneva, Switzerland (2014).
- 44. Immuno-PCR, a new technique for the serodiagnosis of tuberculosis. J. Microbiol. Methods 139, 218–229 (2017).
- 45. Diagnosis of osteoarticular tuberculosis by immuno-PCR assay based on mycobacterial antigen 85 complex detection. Lett. Appl. Microbiol. 74(1), 17–26 (2022).
- 46. Development and evaluation of a multiplex loop-mediated isothermal amplification (LAMP) assay for differentiation of Mycobacterium tuberculosis and non-tuberculosis mycobacterium in clinical samples. PLoS One 16(1), e0244753 (2021).













