Abstract
Various strategies for ocular drug delivery are considered; from basic formulation techniques for improving availability of drugs; viscosity enhancers and mucoadhesives aid drug retention and penetration enhancers promote drug transport into the eye. The use of drug-loaded contact lenses and ocular inserts allows drugs to be better placed where they are needed for more direct delivery. Developments in ocular implants gives a means to overcome the physical barriers that traditionally prevented effective treatment. Implant technologies are under development allowing long-term drug delivery from a single procedure, these devices allow posterior chamber diseases to be effectively treated. Future developments could bring artificial corneas to eliminate the need for donor tissue and one-off implantable drug depots lasting the patient's lifetime.


Scale bar = 100 μm.

Scale bar = 100 μm.
CD: β-cyclodextrin.
Adapted with permission from [35].

The scale used in this image has been exaggerated for clarity.
Ocular drug delivery is hampered by the physiological barriers presented by the eyes. These include blinking and wash out by tears, nasolacrimal drainage, nonproductive losses and impermeability of the cornea [1,2].
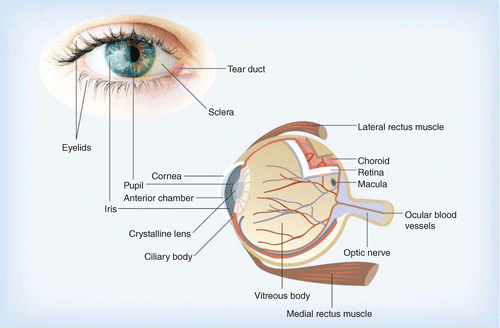
Some of the various structures of the eye are detailed in Figure 1, highlighting the intricate complexity of this organ. The conjunctiva (not shown for clarity) is the mucosa lining the inside surface of the eyelids and the external surface of the front of the eye up to the limbus, the edge of the cornea.
Despite the easy accessibility of the eye for administering medication, in many ways it is an isolated organ with several barriers imposing challenges to drug delivery, tear mechanisms, the physical barriers of its membranes, blood–aqueous and blood–retinal barriers [3].
Topical, systemic and intraocular are the three main routes for administering ophthalmic medication; each has their own advantages and disadvantages. Topical drug delivery is the most accepted route accounting for approximately 90% of aqueous ophthalmic formulations. Advantages are their relative simplicity to formulate, minimal storage limitations and ease of drug instillation by most patients. Disadvantages include limited drug concentration for lipophilic agents, precorneal losses and the barrier function of the cornea [4,5]. For effective systemic delivery a relatively high drug concentration needs to be circulating in the blood plasma in order to achieve a therapeutically effective dose within the eye. Sustained release oral drugs can be suitable for glaucoma patients, allowing for continuous and effective treatment; however, this method exposes the whole body to the drug often giving rise to undesired side effects [6]. Intraocular drug delivery by intravitreal injection is an invasive procedure carrying a degree of risk, such as retinal hemorrhage or detachment, especially if the technique needs to be repeated when treating chronic disorders. However, it is very effective at getting drugs to the posterior segment [3].
The cornea is the main route for topically applied drugs to gain access into the eye and the conjunctival/scleral route can also be efficient [7,8]. Drops are the most accepted means to apply medication to this organ [9]; they are easy to apply by most patients and they are convenient. However, regardless of the ease of access to the eye for topical application of medication, efficient ocular drug delivery is hampered by a series of clearance mechanisms that protect the ocular structures from foreign matter. Upon administration of traditional eye drops they are immediately diluted in the tear film, followed by very quick elimination by the action of blinking, wash out by tears and nasolacrimal drainage [10,11]. After instilling eye drops, there remains a very short time where any residual medication is in contact with the cornea, during which time there is opportunity for the drug to penetrate into the eye; however, due to poor corneal permeability only a very small portion of active pharmaceutical ingredient will be capable of crossing the cornea. Of the applied dose, only 1% or less will successfully reach the intended target in most cases, the rest will be systemically absorbed via the conjunctiva or nasolacrimal mucosa to be eliminated by metabolic processes [5]. The tear film comprises of several compartments; Figure 2 shows the three-layer tear film model comprising of a coating of mucous anchored to the epithelium via microvilli, an aqueous compartment containing soluble mucin and free lipid and a thin lipid layer [11–14].
The tear film and ocular mucosa are the first external barriers to overcome, after which the multilayered structure of the cornea (Figure 3) offers the next challenge; this structure has both lipophilic and hydrophilic properties and there are five distinct layers: epithelium, Bowman's membrane, stroma, Descemet's membrane and endothelium [6,15]. The first corneal layer is the epithelium, which is approximately 50 μm at its center increasing to approximately 100 μm at the limbus; this layer is lipophilic, offering approximately 90% resistance to hydrophilic drugs and approximately 10% to hydrophobic preparations. Immediately underneath the epithelium is the Bowman's membrane, a transitional acellular structure approximately 8–14 μm in thickness. Next we find the hydrophilic stroma; this is a gel-like structure with approximately 80 % water, consisting of collagen, mucopolysaccharides and proteins, and it forms the main bulk of the cornea, some 90 % of its total thickness. Next there is the Descemet's membrane, a tough membrane of approximately 6 μm thickness supporting the endothelium, a single layer of loose, epithelia-like cells important in regulating stromal hydration, and this layer is deposited by endothelial cells. The correct level of hydration is important for the cornea to remain clear and transparent [6,15–16].
The corneal epithelial barrier also has different zones; the basement layer consists of newly formed cells firmly attached to the Bowman's layer, here they are columnar in shape. As new cells are formed the preceding basement cells are pushed forwards, becoming polyhedral in shape; eventually they are moved towards the corneal surface where they become polygonal squamous cells. These superficial epithelial cells have Ca2+-dependent membrane adherent regions; zonula occludens, zonula adherens and desmosomes forming tight junctions [17]. Taken together, these tightly bound cell membrane regions and the lipophilic nature of the epithelium make the structure an extremely efficient barrier that resists intrusion of foreign material, including potentially therapeutic compounds; this creates a major challenge for ocular drug delivery [6,11,18].
Strategies for enhancing ocular drug delivery
Despite traditional eye drops being convenient and simple to use, they are not very efficient and only a small amount of the dose is effectively delivered to its intended target, most is lost due to clearance mechanisms. There are, however, certain strategies that can be employed to improve the bioavailability of drugs. First, solubility enhancers can be used, to improve drug concentrations within the formulation; more medication in the dosage form can mean increased bioavailability. This strategy could allow a smaller droplet to be applied, which would be less susceptible to loss by drainage due to induced reflex tearing and blinking [6]. Second, the formulation can be designed in a form that resists clearance; these dosage forms are retained for a longer period, therefore they have more time to interact with ocular tissue. Next, drug penetration enhancers can be incorporated into the formulation to assist their transit across the cornea [19]. Ocular inserts are another area of active research and development. With this method a drug-loaded device resides in the cul-de-sac under the eyelids or fits directly on the cornea like a contact lens; these devices are often designed with controlled release in mind [20,21]. Drug delivery into the cornea and anterior chamber is difficult enough; delivering an effective therapeutic dose to the posterior segment is a major challenge; in many cases it is not possible to deliver sufficient medication to the posterior structures via the topical route [22]. For diseases of the retina, such as age-related macular degeneration (AMD), diabetic retinopathy and retinitis pigmentosa and related ocular neovascular disease there is often a need to resort to invasive methods for drug delivery. Angiogenesis inhibitor medication via intravitreal injection is an option for getting drugs to the posterior segment, but these are often effective for the short term and need repeat injections, which carries risks such as hemorrhage, endophthalmitis, ocular hypertension and retinal detachment [22–26]. Ocular implants are devices that penetrate the sclera or reside within the deeper ocular structures to deliver drugs for an extended period, sometimes many years, minimizing the need for repeat injections [23]. Implantable devices that are not designed to deliver drugs are also employed to improve the ‘quality of life’ for patients with certain conditions, for example, intraocular lenses. However, drugs to counter postoperative bacterial infection are often included in these devices for short-term protection [27,28]. These various strategies will be discussed in more detail in the following sections.
Solubility enhancers
Discovery of potentially therapeutic compounds is accelerating through developments in genomics, combinational chemistry and the ability to use high-throughput screening. High proportions of newly screened compounds prove to be hydrophobic and are poorly water-soluble [29]. For efficacious performance in the physiological environment drug candidates need to interact within an aqueous media, the interstitial fluids within tissues.
Drugs used for the treatment of ocular disorders often have low aqueous solubility and eye drops are only in contact with ocular tissue for a short time. Formulations that are developed to increase the amount of available drug in solution could improve its bioavailability; therefore, solubility enhancement is an important strategy to use when developing ocular medication. An approach taken by Kulkarni et al. [30] was to take the poorly soluble drug, indomethacin, and convert this drug into its sodium salt. They found that this improved its aqueous solubility and the drug was stable at physiological pH and compatible with excipients used for ocular drug formulation.
Solubility enhancement can be achieved by employing hydrotropic compounds. Evstigneev et al. [31] and Coffman and Kildsig [32,33] reported the effectiveness of caffeine, urea and nicotinamide and its derivatives as efficient hydrotropes for enhancing the solubility of riboflavin, a vitamin with poor aqueous solubility of less than 0.1 mg/ml-1, which is used as a photosensitive drug for the treatment of keratoconus. Cyclodextrins are a class of cyclic supramolecular compounds that have been well studied for dissolution enhancement of low solubility drugs; Loftsson and Stefansson discussed the use of cyclodextrins for complexation with steroids, carbonic anhydrase inhibitors, pilocarpine and cyclosporins in eye drop formulations that are well tolerated [34]. Morrison et al. [35] investigated cyclodextrins for their hydrotropic properties and were able to show that β-cyclodextrin achieved solubility enhancement of more than 140% for riboflavin. Whilst the above mentioned studies achieved modest solubility enhancements, research by Kim et al. [29] investigated the performance of two hydrotropes, N, N-diethylnicotinamide (DENA) and N, N-dimethylbenzamide (DMBA), with 13 poorly water-soluble drugs and these compounds were shown to have superior hydrotropic action – between 1000- and 10,000-fold.
Supramolecular structures are submicron-sized molecules within the realm of nanotechnology and many of these assemblies have solubility enhancement properties. This technology is becoming an important tool within the pharmaceutical industry, with substantial investment within the global market. Dendrimers, microemulsions, nanoparticles, nanosuspensions and liposomes belong to this class of drug delivery vehicles and are proving to be useful structures to improve bioavailability, all of which are at the forefront of research in ocular drug delivery [1–2,36–42].
Micelles are aggregates of amphiphilic molecules forming self-assembled spheres in aqueous media. They have a monolayer ‘shell’ of polar groups with their associated fatty acid ‘tails’ forming the core. These are useful carriers of hydrophobic drugs within the core albeit with limited efficiency owing to a high amphiphile/drug ratio [43]. The work of Qu et al. [44] involved chemical modification of chitosan by increasing the hydrophobicity and this allowed them to produce 100–300 nm-sized micellar clusters that could achieve up to an order of magnitude enhancement in hydrophobic drug bioavailability compared with micelles produced using triblock copolymers. In ocular drug formulations they were able to show an initial prednisolone concentration in the aqueous humor equivalent to that found when using a tenfold dose of prednisolone suspension.
Dendrimers are proving to be useful drug-delivery platforms with an ability to solubilize pharmaceutically active compounds of low aqueous solubility. Dendrimer/drug complexes are able to cross cell membranes and resist premature clearance [45]. Spataro et al. [46] investigated phosphorus-containing dendrimers of generations G0–2 for drug delivery to the ocular posterior segment via topically applied formulations. They considered the quaternary amine core as an analog for benzalkonium chloride (BAC), which is often included in ocular drug formulations as a preservative, whilst the carboxylic acid terminal groups were able to interact with the antiocular hypertension drug carteolol. In vivo studies using the rabbit model showed there was no ocular irritation and despite the low aqueous solubility of the G2 form 2.5-times more carteolol penetrated the aqueous chamber [46].
Penetration enhancement
Materials that modify the corneal epithelia can allow enhancement of drug permeation and this can be achieved using various strategies. BAC is commonly used as a preservative in ocular drug formulations this, together with other compounds (cetylpyridinium chloride [CPC], ethylenediaminetetraacetic acid [EDTA], polyoxyethylene strearyl ether [PSE] and polyethoxylated castor oil [PCO]) are compounds with penetration-enhancing properties. Their mode of action is due to destabilization of the tear film and the protection given by its mucus component (for BAC), and ultrastructural alterations [17] and solubilization of cellular membranes for the other enhancers. Useful as they are for penetration enhancement they can also induce irritation and damage to the ocular epithelium even at low concentrations. Chung et al. [47] and Burgalassi et al. [48] investigated these materials confirming their irritation and cytotoxicity effects. Liu et al. [49] state that penetration enhancers should be:
• Nontoxic;
• Nonirritant to the eye;
• Inert and compatible to other excipients within the formulation;
• Fast acting and reversible action;
• Effective at low concentration.
In their report they discuss the use of several penetration enhancers for ocular drugs – BAC, EDTA, surfactants, heteroglycosides, bile salts, polycarbophil–cysteine conjugates and boric acid – all of which have been used in ophthalmic formulations despite the fact that even at low concentrations they can cause ocular irritation [49]. Morrison et al. [17] investigated drug-penetration enhancement using EDTA and two analogs, EGTA and EDDS, and they found that this was achieved by sequestering Ca2+ and, therefore, loosening tight junctions, which depend on the availability of these ions.
Gelucires are glycerides composed of mono-, di- and triglycerides with mono- and diesters of polyethylene glycol. They are amphiphilic with surface active properties [50]. Gelucire 44/14 has a melting temperature of 44°C and a hydrophilic–lipophilic balance of 14, hence its name. It is a compound known for its permeation-enhancing properties and is ‘generally regarded as safe’ (GRAS). Liu et al. [49] investigated Gelucire 44/14 for its permeability-enhancing performance in vitro and in vivo for various ophthalmic drugs and demonstrated that it enhanced transcorneal permeability of drugs with a range of hydrophilicity/lipophilicity whilst remaining nonirritating. Loftsson and Stefansson [34] reviewed cyclodextrins for enhanced topical delivery of steroids for ophthalmic formulation and the cyclodextrin–drug complexes were found to be well tolerated in eye drop formulations. Cyclodextrins and their drug complexes are too large to partition into the cornea and until recently it was generally thought that they kept the drug in solution at the eye surface where the drug was able to diffuse into the tissue [49,51], or by modulation of the aqueous diffusion layer on the corneal surface [52]. Morrison et al. [35] investigated the use of cyclodextrins as ocular drug delivery excipients for permeability enhancement of riboflavin for the treatment of keratoconus. They have shown that cyclodextrins form complexes with riboflavin and release their drug payload by preferential take up of cholesterol from corneal epithelial cell membranes. The removal of cholesterol renders the epithelium permeable, allowing enhanced drug penetration. Figure 4 shows β-cyclodextrin-induced histological changes to the epithelium of bovine corneas (Figure 4B, D & F), compared with those without cyclodextrin exposure (Figure 4A, C & E). β-cyclodextrin-induced loosening of the epithelium appears to increase with exposure times of 15, 45 and 75 minutes (Figure 4B, D & F, respectively), and this correlates with increased riboflavin penetration without complete destruction of this barrier.
Retention strategies
Precorneal losses have a major impact on ocular drug delivery; it follows that if the drug formulation stays in contact with the intended tissue for longer it is more likely to penetrate the target site to afford its desired action. Adopting an approach for formulation retention is one way to minimize this problem and this can be achieved by several means. Various retention approaches will be discussed in the following section.
Viscosity-enhancing polymers
Natural and synthetic polymers prove useful for their viscosity-enhancing properties in ocular drug formulations for improving residence time. These materials absorb water to form viscoelastic gels, which prove to be suitable vehicles for drug delivery, and they include derivatives of cellulose, poly(vinyl alcohol), poly(vinyl pyrrolidone), carbomers (weakly crosslinked poly[acrylic acids]) and the natural mucopolysaccharide, hyaluronic acid, a component of the vitreous humor [53,54]. Mechanisms for release of incorporated drugs are determined by their chemical structure, network arrangement and swelling properties [55]. Ocular drug delivery formulations incorporating viscosity-enhancing polymers resist lacrimal drainage when residing in the lower conjunctival cul-de-sac. However, disadvantages with this approach are an initial blurring of vision due to changes in refractive index at the corneal surface and difficulty instilling a precise dose [24,56–57].
In situ gelling systems
‘In situ’ gelling systems undergo phase transition from liquid to gel under physiological conditions and this technique has advantages over the simpler viscosity-enhancing systems. Phase transition can be mediated by physiological temperature, pH or electrolyte composition at the cornea surface.
Thermogelling systems include poloxamers [58,59], pluronics and tetronics [60]. Ur-Rehman et al. [61] investigated combined formulations of poloxamer 407 with chitosan as thermogelling delivery systems for ocular, vaginal, orthodontal and parenteral drug administration; this process allowed site-specific tunable drug delivery with enhanced gel strength and mucoadhesive properties. Gratieri et al. [62,63] also worked with poloxamer/chitosan gel-forming systems; their aim was to develop phase-transition gels with improved mechanical and mucoadhesive properties. They investigated poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock polymers (PEO–PPO–PEO) with chitosan at various polymer ratios and found that the polymer/chitosan ratio of 16:1 w/w offered an optimum gelation temperature of 32°C, good resistance to shearing forces at 35°C and good retention due to mucoadhesion. Poly(N-isopropylacrylamide) is a well-researched thermogelling polymer with a lower critical solution temperature (LCST) of 32°C, an ideal temperature for thermosensitive applications for ocular drug delivery, although the polymer precipitates above the LCST forming a stiff gel that can be uncomfortable for ocular drug delivery applications [64]. It also shows reduced transparency above the LCST [65], which would be undesirable for eye drop formulations. Cao et al. [64] investigated thermogelling poly(N-isopropylacrylamide)–chitosan formulation and found it to be a suitable system for ocular delivery of water-soluble drugs, but it is not clear whether they have solved the ‘reduced transparency’ issue with their development. Mayol et al. [59] investigated thermogelling poloxamers (F127 and F68) and found that on their own their gelling properties were not ideal, but could be optimized by the addition of the naturally occurring mucoadhesive polysaccharide, hyaluronic acid. They consider that this approach can be exploited for a range of sustained drug-delivery scenarios and they are especially suited for ocular drug delivery. pH-mediated systems include Carbopol® (Lubrizol Corporation, Ohio) [66], and cellulose acetate phthalate [67]. Electrolyte-triggered gelling systems make the transition from liquid to gel by induction of crosslinking in the gelling system mediated by cations present in the tear fluid, and these include gellan gum (Gelrite®, CP Kelco U.S., Inc), carrageenan [68–70] and sodium alginate [71].
Mucoadhesives
Mucoadhesion is the interaction between a compound, usually a polymer, natural or synthetic, with mucosa or associated mucus [55,72]. Mucoadhesive drug delivery depends on the interplay between the dosage form and mucus-covered mucosal epithelial membranes. Residence time increases owing to this interaction, allowing more time for the drug to penetrate its intended site of action [72,73]. Mucosal adhesion of dosage forms can be explained using a combination of theories [57,74]:
• Electronic theory, where interaction is due to electron transfer between the dosage form and mucosal surface;
• Adsorption theory, where attraction mechanisms are via electrostatic effects, hydrogen bonds and Van der Waals forces. Hydrophobic effects are also implicated, more so when the mucoadhesive polymers are amphiphilic. Covalent bonding can also come into effect between some specific polymers and mucins;
• Wetting theory, which mostly applies to liquid mucoadhesives where there are structural similarities between the polymer and mucin. These effects reduce surface tension and allow the mucoadhesive polymer to spread on the mucosal surface;
• Diffusion theory, which considers the interpenetration of polymer into the mucus and diffusion of soluble mucins into the mucoadhesive.
None of the above mentioned theories can be used to explain mucoadhesion on their own, more, they each play a part to varying degrees within any given scenario [57,75–76]. In considering a typical series of events involving a mucoadhesive–mucosa interaction, first of all the wetting theory comes into play, with wetting and associated swelling of the dosage form; next, physical interactions involving electronic and adsorption theories take place forming noncovalent bonds between the system components; diffusion theory then comes into play when further noncovalent bonds form during interpenetration of polymer–protein chains, during which physical and covalent (chemical) bonds form, again involving electronic and adsorption theories [57,76].
With traditional ocular drug-delivery systems residence time is determined by tear turnover, but for mucoadhesive systems this becomes governed by mucus turnover, hence drug retention and bioavailability is substantially increased [53]. Mucoadhesive polymer films could potentially provide a suitable platform to deliver ocular drugs. Khutoryanskaya et al. [77] investigated the use of complexes and blends of poly(acrylic acid) (PAA) and methylcellulose (MC) to produce polymeric films as vehicles for ocular drug delivery. PAA has excellent mucoadhesive properties owing to an ability to form hydrogen bonds with mucin, although it has limited application for transmucosal drug delivery owing to being very hydrophilic and, thus, quick dissolving. It also has poor mechanical properties and can cause irritation to delicate mucosa. MC has favorable properties that are applied in transmucosal delivery systems; it has excellent biocompatibility profiles but poor mucoadhesive properties. The researchers used a polymer blend approach with different combinations of PAA/MC under a range of pHs and optimized a formulation bringing together the favorable properties of both polymers. In vitro studies of drug-loaded polymer films determined their release profiles and they found that films enriched in MC had significantly slower drug-release profiles than films enriched in PAA. This could allow a tunable drug-delivery system depending on whether rapid or sustained release is required. They further investigated in vivo retention of the polymer films using rabbits and found that 100% MC films were retained for up to 50 minutes, but successful application was hampered by poor mucoadhesive properties. 100% PAA films were strongly mucoadhesive but retention was poor owing to quick dissolution. They concluded that polymer blends had good bioadhesive qualities and showed better retention of 30–60 minutes compared with the films composed of individual polymers [77].
Nanoparticles
Nanoparticle drug-delivery systems are more generally described as submicron-sized structures; these systems were described by Nagarwal et al. [19] as 10- to 1000-nm particles in which drugs could be loaded by attachment to the matrix or dissolved within, encapsulated or entrapped within the structure giving a versatile drug-delivery system. Hans and Lowman [78] discuss biodegradable polymeric nanoparticles for drug delivery, they suggest that surface modified biodegradable solid nanoparticles have an advantage regarding controlled release, principally for targeted drug delivery for the treatment of specific organs, in particular for extended drug delivery to the cornea and conjunctiva [78]. Ibrahim et al. [79] describe a mucoadhesive nanoparticle system as a carrier for gatafloxacin/prednisolone biotherapy for treatment of bacterial keratitis, a serious corneal condition that could lead to blindness without rapid and appropriate intervention. The drug-loaded nanoparticle systems they describe were produced from Eudragit® RS 100 and RL 100 (Evonik Industries AG, Germany) and were coated with the bioadhesive polymer, hyaluronic acid. Nanoparticles within the suspensions produced using these systems were in the range of 315 nm to 973 nm. For ocular drug delivery, supramolecular structures, complexes and composites belong to nanoparticulate systems and these can include microemulsions, liposomes, niosomes, dendrimers and cyclodextrins [1–2,36–41]. Kassam et al. [80] investigated the use of nanosuspensions for ophthalmic delivery of three virtually insoluble glucocorticoid drugs in aqueous media; hydrocortisone, prednisolone and dexamethasone. Their findings show an enhancement to the rate and extent of ophthalmic drug absorption together with improved drug performance compared with aqueous solutions and microcrystalline suspensions. De Campos et al. [81] investigated the interaction of poly(ethylene glycol) (PEG)- or chitosan-coated colloidal nanocapsules with ocular mucosa; they concluded from ex vivo studies that the systems they developed enhanced permeation of dye through the cornea. Evidence from confocal microscopy shows their systems penetrated the epithelium of rabbit cornea via the transcellular pathway and they found that PEG-coated colloids had an enhanced rate of transport across the whole epithelium, whilst chitosan-coated nanocapsules were retained in the superficial epithelial layers. They suggest these systems could be designed as colloidal drug carriers targeting a specific purpose, that is, to attach to the cornea or penetrate into or through it. This implies these systems should prove useful in treating conditions of the cornea and deeper structures within the eye.
Irmukhametova et al. [82] reported the development of fluorescently-labeled sub-100 nm thiolated nanoparticles that showed enhanced retention on bovine cornea in vitro. This retention was related to the formation of disulfide bridges between SH-groups present on the surface of these nanoparticles and cystein-rich domains in ocular mucins. Once these nanoparticles were PEGylated their retention potential was lost owing to lack of SH-groups and screening effects caused by PEG. Although these silica nanoparticles were not loaded with any drug, they served as a good model to study the effect of particle functionality on precorneal retention.
Diseases of the posterior section of the eye include macular degeneration, diabetic retinopathy, retinitis pigmentosa and related ocular neovascular disease. Topical delivery of drugs to the posterior section of the eye is particularly challenging owing, not least, to ocular barrier function and internal clearance mechanisms within the anterior chamber. Recent developments in the field of nanoparticles involve submicron-sized liposomes (ssLips) and these are proving useful for topical drug-delivery systems in the form of eye drops for the treatment of posterior segment diseases. Studies by Hironaka et al. and Inikuchi et al. [83,84] showed successful delivery of coumarin-6 to the retina via noncorneal and nonsystemic pathways using eye drops.
Ocular inserts
Ocular inserts are drug-loaded devices placed in the upper or lower cul-de-sac and in some cases, directly on the cornea; their purpose is to act as a controlled-release drug reservoir. These systems can be insoluble devices that need to be removed after a given period of time or they can be designed to dissolve, erode or biodegrade at the ocular surface. Early forms of ocular inserts have been used since the Middle Ages and were given the Arabic term al-kohl. By the 19th century, paper patches soaked with drug solutions were used and in the early 20th century glycerinated gelatin systems were in use [85]. It is not clear how effective these early devices were, however, drug delivery by this means has developed and devices can be soluble ophthalmic drug inserts (SODIs) or insoluble polymers, mucoadhesives or soluble natural materials such as collagen (e.g., porcine sclera collagen shield bandage lenses) [4]. Ideally these devices could be applied and left in place with no further intervention thereafter. Ocular inserts need to be discreet and comfortable to gain patient acceptance. Sustained-release ophthalmic inserts are defined as sterile devices that can be drug-impregnated thin, single or multilayered films, solid or semisolid materials. The objective being to extend ocular contact time, thus, improving bioavailability. Development of ocular inserts that bring reliable controlled-release drug delivery and patient comfort offers a considerable challenge. The main classes of devices are insoluble, soluble and biodegradable inserts [86]. Ocusert® (Alza Corporation, Palo Alto) was the first relatively successful product for delivery of pilocarpine for the treatment of ocular hypertension and has been commercialized since 1974. Ocusert® consists of a pilocarpine–alginate reservoir sandwiched between thin ethylene–vinyl acetate films; the devices are designed to deliver pilocarpine at either 20 µg/h or 40 µg/h. Some disadvantages of this system were unreliable control of intraocular pressure, leakage, folding, difficulty inserting the devices and ejection or irritation [85,87]. Ocufit SR® (Escalon® Medical Corp) are sustained-release rod-shaped devices made from silicone elastomer, designed to reside in the lower conjunctival fornix; these devices are well tolerated and expulsion is significantly less than with oval or flat inserts. Minidisc ocular therapeutic system (OTS) by Bausch & Lomb (UK) are drug-loaded polymer discs with a similar shape to contact lenses, but are smaller (4–5 mm); they were designed to reside on the sclera in the upper or lower fornix and deliver the antibiotics gentamicin or sulfisoxazole between 3–14 days depending on the system. The company produces nonerodible hydrophobic and hydrophilic systems and erodible devices based on hydroxypropyl cellulose. The inserts are comfortable and easy to use for most patients. Smith & Nephew Pharmaceutical Ltd (UK) patented what they term ‘new ophthalmic delivery system’ (NODS®); these devices offer precision pilocarpine delivery for glaucoma patients from poly(vinyl alcohol) (PVA) film flags. These devices attach to the mucosal surface of the lower conjunctival sac where it takes up fluid from the tears, swells and delivers its drug payload at a predetermined rate into the lacrimal fluid as it slowly dissolves [85]. Mydriasert® are insoluble devices marketed by IOLTech (Germany) for the delivery of phenylephrine and tropicamide to induce sustained mydriasis during surgery or for examination of the fundus (interior ocular surface) [3].
Human amniotic membrane has been used for corneal transplant to treat corneal disorders and ulcerative ocular conditions and for use as bandage lenses. Resch et al. [88,89] investigated its use as a drug-loaded ocular device to deliver ofloxacin in vitro and they concluded that single layer human amniotic membrane had a significant reservoir capacity capable of delivering the drug for up to 7 h in vitro. They propose that drug pretreatment of amniotic membrane could be beneficial when using this tissue for ocular transplant when treating infectious keratitis [88,89]. Table 1 lists some advantages and disadvantages for using ocular inserts [20,85,90].
Recent developments in ocular insert drug-delivery systems
Colo et al. [91] investigated the effect of adding chitosan hydrochloride (CH-HCl) to mucoadhesive erodible ocular inserts produced from poly(ethylene oxide) (PEO) of various molecular weight for delivery of ofloxacin. They added 10, 20 and 30% medicated CH-HCl microparticles to PEO formulations made from 900 kDa or 2000 kDa. Erosion of the devices was accelerated proportional to CH-HCl content. The lower molecular weight PEO proved more suitable for prolonged drug release. They concluded that inclusion of CH-HCl in the devices aids erosion and enhances corneal permeability of ofloxacin when compared with devices not containing CH-HCl. Hornof et al. [92] developed mucoadhesive devices based on thiolated PAA and these were evaluated in human in vivo studies. Their aim was to develop mucoadhesive ocular inserts for controlled delivery of ophthalmic drugs using fluorescein as a fluorescent tracer to determine release rates from the devices in humans. They compared mean fluorescein concentrations in the tear film and cornea as a function of time after instillation of eye drops and inserts composed of thiolated and unmodified PAA. The thiolated polymer inserts formed a soft, insoluble hydrogel and were well tolerated by volunteers. Their findings show this material offers a promising platform for ocular drug delivery for a prolonged duration. Mishra and Gilhotra [66] designed and characterized a bioadhesive in situ gelling ocular insert for the delivery of gatifloxacin using a mixture of sodium alginate with chitosan, which was plasticized with glycerin. They combined sodium alginate for its gelling properties, with chitosan for its bioadhesive qualities, formulations of various proportions were prepared and films were produced using the solvent casting technique as described by Pandit et al. [93]. Using this system they found an accumulative drug release of 95–99% during 8–12 h and the formulation consisting of 2% alginate with 1% chitosan had the most sustained release of 12 h. They concluded that this system allowed production of uniform in situ gelling polymer films suitable for controlled release of gatifloxacin for the treatment of bacterial keratitis and conjunctivitis [66]. Natamycin is a polyene antibiotic used for the treatment of fungal blepharitis, bacterial keratitis and conjunctivitis and it has the ability to reduce intraocular pressure. Rajasekaran et al. [94] compared the controlled-release performance of natamycin from ocular inserts they designed from a variety of polymeric materials; Eudragit® L-100, S-100, RL-100, hydroxypropyl methyl cellulose phthalate (HMCP) and cellulose acetate phthalate (CAP) in different proportions with PEG-400 as a plasticizer. Their aim was to develop devices for in situ sustained drug delivery and their approach was to prepare polymeric films using the solvent casting method. 1-cm discs were cut from the films to be used as inserts; these were evaluated for their physicochemical properties, such as drug concentration, weight, folding durability, thickness, moisture absorption and vapor transmission rate. FTIR studies established that there was no chemical interaction between the drug and polymers used. In vitro studies were conducted to determine their drug release kinetics; devices made from CAP, HPMCP and Eudragit® S-100 released all of their drug payload within 10–15 h, whilst inserts made from increased concentrations of Eudragit® RL-100 continued release for 18–23 h; best performance was shown for formulations consisting of 3% Eudragit® RL-100 and 1% Eudragit® L-100. They concluded that natamycin-loaded ocular inserts produced from 3% Eudragit® RL-100 and 1% Eudragit® L-100 plasticized with 33% PEG-400 are capable of controlled drug delivery up to 23 h [94].
Contact lenses for drug delivery
Contact lenses are hard or soft polymeric devices designed to fit directly onto the cornea to correct refractive abnormalities; they can be produced from hydrophilic or hydrophobic polymers. Hydrogel contact lenses are realistic products to act as ocular drug-delivery systems; they are able to imbibe a large volume of aqueous solution relative to their anhydrous form. If the aqueous solution that hydrates the contact lens contains sufficient pharmaceutically active material this will be able to diffuse from the polymer matrix into the tear film bathing the eye and subsequently interact with the ocular tissue. However, there still remains a need to retain the drug within the devices sufficiently to provide sustained release.
The idea of using hydrogel contact lenses as drug-delivery devices was first suggested by Wichterle et al. [29,95] in their 1965 patent, in which they suggest the inclusion of medication upon lens hydration to offer extended drug availability during wear. Contact lens design determines how they are to be used; daily, weekly and monthly disposable options are available [95]. Early approaches to contact lens-aided drug delivery relied on absorbance of drug-loaded solution during prewear soaking. Conventional contact lenses have limited drug-loading potential and drug delivery using this method proves unreliable, giving an initial ‘burst release’ followed by rapid decline over a relatively short period [20,96]. Other methodologies include molecular imprinting technology, drug-loaded coating or addition of a sandwich layer of drug-loaded polymer, inclusion of drug-loaded nanoparticles and cyclodextrin grafting [28]. Molecular imprinting technology is a technique whereby the polymer formulation is modified to give it a higher affinity towards drug molecules, thus, increasing their drug-loading potential and prolonging delivery [97–99]. Hiratani et al. [96] took this approach in developing a system employing methacrylic acid, N, N-diethylacrylamide and the drug timolol; from this system they were able to achieve sustained timolol release for almost 48 h in vitro. Alvarez-Lorenzo et al. [100] applied the same strategy to produce norfloxacin-loaded poly(hydroxyethyl methacrylate) (pHEMA) contact lenses and they report that reservoir capacity was enhanced by up to 300-fold compared with pHEMA lenses without molecular imprinting technology. Hyatt et al. [101] investigated the release profiles of gentamicin and vancomycin from fibrin-coated and fibrin-sandwiched contact lenses in vitro; their aim was to develop a system that could offer controlled and sustained drug delivery for a minimum period of 8 h. They concluded that the fibrin gel/lens systems performed better for extended delivery of gentamicin compared with normal lenses soaked with the antibiotic solution, however, their performance for delivering vancomycin was poor compared with soaked lenses. Lenses incorporating fibrin showed potential for treating microbial keratitis. Ciolino et al. [102,103] investigated poly(lactic-co-glycolic acid) (PLGA) coatings and sandwiched films with contact lenses as potential drug-delivery devices. They found that contact lenses incorporating PLGA film retained antifungal properties up to 3 weeks in vitro, and their prototype ciprofloxacin-eluting contact lens demonstrated controlled release at therapeutically active concentrations for up to 4 weeks in vitro. Although fibrin- or PLGA film-sandwiched and coated lenses bring sustained drug delivery benefits, the lenses are opaque; therefore they require a clear ‘window’ in the centre of the lens allowing the patient to see during treatment [100–103]. Inclusion of drug-loaded nanoparticles within the polymer matrix of the contact lens is an effective strategy for prolonged drug delivery. This approach can allow sustained release, which can be tuned towards the patient's needs, anything between a few hours to several weeks. Gulsen and Chauhan [104] conducted a pilot study to determine the effectiveness of nanoparticle-laden pHEMA. The nanoparticles were based on oil-in-water microemulsion loaded with lidocaine, a hydrophobic drug; the droplets were then encapsulated in a silica shell, which stabilized the nanoparticles and these were incorporated in the hydrogel matrix during polymerization. Hydrophobic lidocaine has a slight and finite solubility in water; therefore it is able to slowly diffuse from the nanoparticles into the aqueous phase of the gel matrix where it would then be able to further diffuse into the tear film. The nanoparticle-laden hydrogels remained clear and drug release studies in vitro showed an initial burst release followed by slow and steady release thereafter; by day 10 virtually all the drug had been released. They concluded that the nanoparticle-loaded hydrogels could be suitable for controlled drug delivery for several days at therapeutically effective concentrations. Gulsen and Chauhan [105] followed up their previous investigation of nanoparticle-laden pHEMA by developing four more microemulsion-based formulations, type 1 and 2 were based on canola oil with Tween® 80 and Panadon SDK, with or without a stabilizing silica shell, and type 3 and 4 were based on hexadecane with Brij® 97 with or without a stabilizing silica shell; they incorporated lidocaine as a model drug. The type 1 formulation was opaque due to the poor solubility of Tween® 80 in HEMA, the type 2 formulation lost some transparency but was not opaque indicating that the silica shell reduced interaction between the surfactant and HEMA. Type 3 showed minimal transparency reduction but was not as transparent as pHEMA, type 4 showed no observable loss of transparency due to stabilization afforded by the silica shell. Release studies in vitro determined that formulations based on hexadecane with Brij® 97 were suitable for sustained drug delivery at therapeutic rates for up to 8 days, the Tween® 80-based formulation was deemed unsuitable due to poor stability and particle aggregation. Gulsen and Chauhan speculate that furthering this work to develop ‘smart’ particulate-based systems that could respond to pH or temperature change could minimize burst release and decaying release rates [104,105]. The approach followed by Jung and Chauhan [106] was to develop a timolol-loaded nanoparticle/HEMA-based contact lens system. Their aim was to produce nanoparticles without using surfactant owing to opacity issues when these are used with HEMA. Using thermal polymerization techniques they formed drug-loaded nanoparticles based on crosslinking monomers, propoxylated glycerol triacrylate (PGT) and ethylene glycol dimethacrylate (EGDMA), and incorporated these in pHEMA hydrogels. Their product was a transparent drug-loaded hydrogel with temperature-dependent release rates between 2–4 weeks. They concluded that their system maintains drug stability under refrigerated conditions and the temperature change promotes drug release upon insertion of the lenses into the eyes. Figure 5 shows how nanoparticles could release entrapped drug molecules into the pre- and post-tear films.
Drug-loading capacity of hydrogel contact lenses can be enhanced by the inclusion of ‘container molecules’. Cyclodextrins, with their ‘guest–host’ properties, have been investigated for this purpose. Complexation between cyclodextrins and drug molecules is a dynamic process due to the weak noncovalent interactions in play. The strategy followed by dos Santos et al. [107] was to synthesize methacrylated β-cyclodextrin and use it to form copolymer with HEMA and EGDMA; the polymers formed had clear gel properties. Drug loading was achieved by soaking the anhydrous polymers in solutions of acetazolamide or hydrocortisone for 4 days. The performance of these methacrylated β-cyclodextrin hydrogels was studied in vitro and they were found to offer tunable drug-loading/release rates with capacity for sustained drug delivery over several days. They followed up this study with development of another hydrogel formulation using β-cyclodextrin grafted onto pHEMA-co-glycidyl methacrylate (GMA). This system was able to enhance diclofenac loading by 1300% and could sustain drug release for 2 weeks in lacrimal fluid. They concluded that these systems could have potential for pharmaceutical applications in soft contact lenses and other medicated devices [108]. Xu et al. [109] produced hydrogel films and contact lenses from HEMA, mono-methacrylated β-cyclodextrin and trimethylolpropane trimethacrylate. Puerarin was incorporated as a model drug by soaking in drug solution to hydrate the gel. In vitro studies determined loading and release rates were dependent on β-cyclodextrin content. In vivo studies using rabbits showed the gels offered sustained drug release with superior performance compared with commercial puerarin eye drops. The devices had excellent mechanical properties and the researchers propose the material is suitable for drug delivery from reusable daily wear contact lenses.
Ocular implants
Treating the posterior segment
Historically, the posterior segment has been exceptionally difficult to treat owing to the many barriers that obstruct ingress of foreign matter into the eye. The development of ocular implants has allowed these external barriers to be overcome. Modern devices allow long-term treatments for otherwise impossible to treat conditions, and many devices provide medication for years from a single procedure [110,111].
Drug-eluting intraocular lenses
Intraocular lens (IOL) surgery is a well-established and safe procedure routinely performed worldwide; however, as with any surgical technique there is always risk from infection or other complications, for example, postoperative inflammation, posterior capsule opacification (PCO) and secondary cataracts caused by epithelial cell adhesion and proliferation in the posterior lens capsule. Introduction of preventative medication during surgery is subject to decay or elimination before it can be effective. Much research is currently carried out for the development of drug-eluting IOLs to minimize postoperative problems and also to address concurrent pathologies. IOL/drug combinations can be achieved by preinsertion soaking in concentrated drug solution (only useful for drugs with a high affinity for the polymer), coating with layers of drug/polymer, chemical grafting of drugs, drug impregnation using super critical fluids and attaching inserts onto the haptics (the ‘arms’ of the IOL) [28]. A study by Kleinmann et al. [112] determined that commercial hydrophilic acrylic lenses (C-flex, Rayner intraocular lenses) [113] have affinity for fourth generation fluoroquinolones and were able to release this drug above the minimum inhibitory concentration in rabbits for at least 12 h. They concluded that C-flex/drug combination is safe and effective for delivery of these antibiotics. Davis et al. [114] investigated concentrations of four antibiotics (moxifloxacin, gatifloxacin, linezolid and ceruroxime) in aqueous and vitreous humor samples from rabbit eyes. Drug released from implanted hydrophilic IOLs was analyzed using HPLC to determine drug concentration in the ocular fluid samples. The IOLs used were STAAR Nanoflextm Collamer® (USA), 40% water content material comprised of a collagen, pHEMA blend [115], presoaked in antibiotic solution. Ocular fluid samples were taken for analysis at intervals up to 24 h. It was established that the antibiotics studied were above the minimum inhibitory concentration in the aqueous humor for at least 6 h, notably, gatifloxacin concentrations remained above this level at 24 h after implantation [115]. Layer-by-layer deposition is a technique used for coating opposing charge polymers to rigid hydrophobic IOLs, a drug can be incorporated during this process. Coating pHEMA-based hydrophilic IOLs by immersion in octadecyl isocyanate can be an effective method to give controlled release from norfloxacin-containing IOLs. Grafting drug molecules onto the IOL surface can provide a permanently active surface to prevent cell adhesion, or allow release of drugs by some external trigger, for example, light irradiation. High drug concentrations within a polymeric matrix can be achieved using supercritical CO2 as a means to force drugs into the polymer without the need for organic solvent [28]. Duarte et al. [116] employed supercritical CO2 technology to impregnate p(MMA-EHA-EGDMA), a suitable polymer for IOL manufacture, with flurbiprofen, an anti-inflammatory drug used for intraocular delivery. Their experiments found the process allowed higher drug impregnation and release studies showed the system to be effective for up to 3 months. The approach employed by Garty et al. [27] was to produce norfloxacin-loaded pHEMA cylinders in 1.0 mm diameter microglass tubes with 0.09 mm stainless steel wire through the centre during room temperature polymerization. When fully polymerized the hydrogel was ejected from the tube and the wire removed leaving a tubular hydrogel structure, this was washed with sterilized water to remove unreacted components. The gel was cut into 1.0 mm lengths and lyophilized. Next they added a hydrophobic coating using octadecyl isocyanate to control drug release. The devices were used as sleeves placed over IOL haptics and this assembly was used in lens-replacement procedures in the rabbit model. Results from in vivo studies showed the devices offered sustained drug delivery above the minimum inhibitory concentration for over 4 weeks. They conclude that these controlled-release devices are effective at sustained delivery of therapeutic levels of drugs within the anterior chamber postoperatively. Incorporation of drugs with IOLs has predominantly aimed at postoperative delivery of antibiotics and anti-inflammatory medication [27].
Drug delivery by intravitreal injection
There are many debilitating and sight threatening conditions resulting from posterior segment diseases and in most cases the only way these can be treated is by invasive procedures, for example, ‘intravitreal injection’. In the main this still remains so, however, developments have brought a diverse range of effective implantable drug-delivery systems targeting posterior segment disease and the various options will now be considered [22] The most common means to place drugs in the posterior chamber employs injection into the vitreous humor; this provides a high concentration of drug where it is needed and minimizes systemic complications. Xu et al. investigated the diffusion of polystyrene nanoparticles of various size and surface chemistries in fresh bovine vitreous and they were able to achieve tuneable drug transport within the posterior chamber depending on the designed properties of the nanoparticle [117]. However, many conditions require repeated treatment and this can cause intraocular problems, for example, cataract, retinal detachment, hemorrhage, endophthalmitis and ocular hypertension.
Intraocular implants
In an attempt to overcome the problem of frequent injections biodegradable and nonbiodegradable drug depot devices that can offer long-term drug release into the posterior chamber have been developed and further research in this area is ongoing. Solutions, liposomes, micelles, nanoparticles and vectosomes are suitable for intravitreal injection, although these dosage forms only give short-term drug availability, generally days to several weeks [23,118]. Biodegradable and nonbiodegradable drug depot devices have been developed and further research in this area is ongoing. Implantable devices for long-term drug delivery are on the market or currently undergoing clinical trial. Vitrasert® (Control Delivery Systems, Inc., now pSiveda Corp.) is a drug depot device for sustained delivery of ganciclovir via a rate-limiting PVA/ethylene vinyl acetate (EVA) membrane for up to 8 months [22,118–119]. Retisert® intraocular inserts (pSiveda Corp.) were approved by the US FDA in 2005. They are inserts for delivery of the corticosteroid fluocinolone acetonide for treatment of posterior uveitis, a serious sight threatening condition. The devices are designed for long-term drug release up to 30 months [120]. Vitrisert® and Retisert® inserts are nondegradable and require surgical implantation and removal [22]. Medidur® (Alimera Sciences, Inc., Alpharetta, GA/pSivida Inc.) are implantable devices for delivering fluocinolone acetonide for up to 36 months. This device consists of a narrow cylindrical polyimide tube loaded with the drug and PVA-based end caps provide rate-limiting drug delivery. The 3.5-mm long device is inserted through a 25-g needle carried out under local anesthesia and creates a self-healing wound eliminating the need for surgery [121]. Implants employing biodegradable polymers are promising systems for intraocular drug delivery. Sivaprasad et al. [122] report the use of the Posurdex® biodegradable polymer device for treatment of macula edema using dexamethasone. This drug has a half-life of less than 24 h, therefore it provides only limited management of this condition by injecting the drug. However, dexamethasone-containing Posurdex® devices were shown to deliver the drug at a constant rate for up to 4 months, these devices have been renamed Ozurdex® and are marketed by Allergan Inc. (USA) [123]. In vivo studies using monkeys showed the system was effective at reducing retinal vasculopathy and neuropathy [124]. Surodex® (Oculex Pharmaceuticals) is a poly(lactic-glycolic acid) device to be inserted in the anterior or posterior chamber at the time of cataract surgery to deliver dexamethasone for up to 10 days. Tan et al. [125] conducted a randomized clinical trial to evaluate the effectiveness of the Surodex® insert as a safe and effective treatment of intraocular inflammation in post-cataract surgery. Their study employed flare meter readings to determine inflammation and this showed that measured values were lower in all readings from the Surodex® group compared with those treated postoperatively with dexamethasone eye drops. They concluded that implantation of a single Surodex® device at the time of cataract surgery reduces post-surgery inflammation [126,127].
Future perspective
In this review the various strategies for enhancing bioavailability of ophthalmic drugs have been considered; how drug bioavailability can be improved using solubility, retention and permeability enhancers has been explored. Drug-loaded contact lenses allow localized delivery directly to the cornea, where the lenses offer controlled release whilst isolating the postcorneal tear film from lachrymal clearance. Nanoparticle technology is allowing drug delivery to the posterior chamber via topically applied formulations. Future research is likely to bring discoveries of materials with superior performance compared with those in current use and these could include smart drug-delivery systems that release their payload in response to a stimulus, e.g., light.
The use of ocular inserts for extended and intimate contact between the dose form and ocular tissue proves to be a beneficial strategy and the use of ocular implants allows all external barriers to be overcome, giving direct access to internal tissues whilst minimizing side effects. Many of these approaches have been developed in recent decades and continue to be improved upon with new innovations. Looking to the future, innovative advances to delay or prevent blindness could be made; developments in two main areas could be speculated; the cornea and vitreous humor. First, corneal disease has a major influence on visual health; corneal tissue-engineered constructs are being developed to test new ocular drugs. Future development of artificial corneas could become a possibility to replace diseased ones without the need for donor tissue, which is a scarce commodity [127,128]. Another area for advanced drug delivery is the posterior segment; vitrectomy is an invasive but well-established procedure for many posterior segment disorders. A synthetic material is used to replace natural vitreous humor. The possibility of developing synthetic materials for whole or partial vitrectomy as a drug depot could allow long-term controlled release for decades. A one-off procedure would be more favorable than many less effective ones over the course of a lifetime [129,130].
| Advantages | Disadvantages |
|---|---|
|
|
Key Terms
Ocular insert: A drug-loaded device designed to reside within the ocular cul-de-sac, attach to the conjunctiva or directly onto the cornea.
Ocular implant: Dosage forms implanted directly into the ocular globe; these can be devices that bring ‘quality of life benefit’, such as intraocular lenses used for crystalline lens replacement. Implantable devices are also used for sustained and controlled drug delivery to the posterior segment.
Hydrotrope: Water-soluble compound that improves the aqueous solubility of hydrophobic or poorly water-soluble compounds.
In situ gelling system: Liquid formulations that turn into gel upon dosage form administration. These phase transitions can typically be triggered by changes in temperature, pH or electrolyte interaction.
Mucoadhesive: A compound, usually a polymer, with the ability to adhere to mucosal tissue.
Bandage lens: Device designed to fit directly onto the front of the eye to offer protection during the healing process, for example, after corneal surgery.
Container molecule: Molecular structures with cavities that can accommodate another molecule via guest–host complexation.
‘Smart’ drug-delivery system: Responsive drug-delivery systems where a favorable change takes place in response to some form of stimulus, for example, change in temperature, pH, ionic interactions or stimulation from a light source.
Executive Summary
Strategies to enhance the bioavailability of drugs are:
Drug solubility & penetration enhancement
• Many ocular drugs have low aqueous solubility; this can be improved using hydrotropic compounds. Formulating for higher drug concentration means increased availability.
• Inclusion of penetration enhancers within a formulation improves drug partitioning into tissue.
Drug retention strategies
• Viscosity-enhancing polymers, in situ gels and bioadhesives allow eye drop formulation to resist precorneal losses and they retain intimate contact with ocular tissue longer giving the dose form more time to penetrate ocular membranes.
• Drug delivery from ocular inserts are a means to place the dose form in immediate contact with ocular mucosa, this strategy allows controlled and sustained drug release for an extended period.
Ocular implants
• Implantable devices are designed to penetrate the ocular membranes or reside entirely within the eye. This strategy overcomes all external barriers and can offer short term medication or deliver medication for several years when treating chronic conditions.
Future perspective
• A speculative outlook considered the possibility of innovative technologies developing synthetic tissues to enable testing new drugs and possibly even produce artificial corneas for transplant. The idea of developing novel materials for vitreous humor replacement as lifetime drug delivery depots could potentially be realized.
Financial & competing interests disclosure
The authors wish to thank BBSRC for doctoral research funding to PWJM (BB/F017189/1). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as; • of interest. •• of considerable interest
References
- 1 . Cationic gelatin nanoparticles for drug delivery to the ocular surface: in vitro and in vivo evaluation. J. Nanomater. 238351 (2013).(2013)
- 2 . Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 6(2), 324–333 (2010).
- 3 . Recent advances in intraocular drug-delivery systems. Recent Pat. Drug Deliv. Formul. 5(1), 1–10 (2011).
- 4 . Ophthalmic drug-delivery systems – recent advances. Prog. Retin. Eye Res. 17(1), 33–58 (1998).
- 5 . Drug-delivery systems for enhanced ocular absorption. In: Enhancement in Drug Delivery. Touitou E, Barry BW (Eds). (CRC Press, FL, USA, 489–525 (2007). Eds).
- 6 . Ocular drug delivery. In: Physiological Pharmaceutics: Barriers to Drug Absorption, 2nd ed. CRC Press, FL, USA, 249–270 (2001).
- 7 . Drug delivery to the eye: special reference to nanoparticles. Int. J. Drug Deliv. 2, 12–21 (2010).
- 8 . Penteration into the anterior chamber via the conjunctival/scleral pathway. J. Ocul. Pharmacol. Ther. 13(1), 41–59 (1997).
- 9 . Effect of pH and formulation variables on in vitro transcorneal permeability of flurbiprofen: a technical note. AAPS PharmSciTech 9(3), 1031–1037 (2008).
- 10 . Comparative review on conventional and advanced ocular drug delivery formulations. Int. J. Pharm. Pharm. Sci. 2(4), 1–5 (2010).
- 11 . Ocular Penetration Enhancers. In: Enhancement in Drug Delivery. Touitou E, Barry BW (Eds). (CRC Press, FL, USA, 527–547 (2007). Eds).
- 12 . Eye structure and physiological functions. In: Enhancement in Drug Delivery. Touitou E, Barry BW (Eds). (CRC Press, FL, USA, 473–487 (2007). Eds).
- 13 . Functional aspects of the tear film lipid layer. Exp. Eye Res. 78(3), 347–360 (2004).
- 14 . A compositional based model for tear the film lipid layer. Trans. Am. Ophthalmol. Soc. 95, 79–88 (1997).
- 15 . Ophthalmic drug delivery. In: Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists. Hillery AM, Lloyd AW, Swarbrick J (Eds). (CRS Press, FL, USA, 329–353 (2001). Eds).
- 16 . Prodrugs for improved ocular drug delivery. Adv. Drug Deliv. Rev. 19(2), 203–224 (1996).
- 17 . Enhancement in corneal permeability of riboflavin using calcium sequestering compounds. Int. J. Pharm. 472(1–2), 56–64 (2014).
- 18 . Identification of a Na+-dependent cationic and neutral amino acid transporter, B0,+, in human and rabbit cornea. Mol. Pharm. 1(5), 338–346 (2004).
- 19 . Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J. Control. Release 136(1), 2–13 (2009).
- 20 . Ocular inserts – advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 1(3), 291–296 (2010).
- 21 Hydrogel contact lens for extended delivery of ophthalmic drugs. Int. J. Polym. Sci. 1–9 (2011).(2011).
- 22 . Current and future ophthalmic drug-delivery systems: a shift to the posterior segment. Drug Discov. Today 13(3–4), 135–143 (2008).
- 23 . Drug delivery to the posterior segment of the eye. Drug Discov. Today 16(5–6), 270–277 (2011).
- 24 . Recent developments in ophthalmic drug delivery. Pharm. Sci. Technol. Today 1(8), 328–335 (1998).
- 25 . Current and potential therapies for ocular neovascular diseases. Clin. Exp. Pharmacol. 3(4), (2013).
- 26 . Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release 136, 247–253 (2009). •• Designed a topical drug-delivery system using submicron-sized liposomes as carriers targeting the retina from a noninvasive system.
- 27 Sustained antibiotic release from an intraocular lens-hydrogel assemble for cataract surgery. Invest. Ophthalmol. Vis. Sci. 52(9), 6109–6116 (2011). •• Developed a drug-delivery device designed to attach to the haptics of intraocular lenses, potentially useful for inclusion of other drugs for post-surgery.
- 28 . Drug-eluting intraocular lenses. Materials 4, 1927–1940 (2011).
- 29 . Hydrotropic solubilisation of poorly water-soluble drugs. J. Pharm. Sci. 99(9), 3953–3965 (2010).
- 30 . Solubility enhancement of water insoluble drug for ophthalmic formulation. Int. J. Drug. Deliv. 3, 141–148 (2011).
- 31 . Effect of a mixture of caffeine and nicotinamide on the solubility of vitamin (B2) in aqueous solution. Eur. J. Pharm. Sci. 28(2), 59–66 (2006).
- 32 . Effect of nicotinamide and urea on the solubility of riboflavin in various solvents. J. Pharm. Sci. 85(9), 951–954 (1996).
- 33 . Hydrotropic solubilisation – mechanistic studies. J. Pharm. Res. 13(10), 1460–1463 (1996).
- 34 . Cyclodextrins in eye drop formulations: enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 80, 144–150 (2002).
- 35 . Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol. Pharm. 10, 756–762 (2013). • Developed methods to enhance drug solubility and corneal permeability. Proposes a mechanism for corneal epithelial permeability enhancement using cyclodextrin.
- 36 . Nanotech approaches to drug delivery and imaging. Drug Discov. Today 8(24), 1112–1120 (2003).
- 37 . Nanotechnology in ocular drug delivery. Drug Discov. Today 13(3–4), 144–151 (2008).
- 38 . Nanoparticles laden in situ gel for sustained ocular drug delivery. J. Pharm. bioallied Sci. 5(2), 162–165 (2013).
- 39 . Nanoparticles in ocular drug delivery. Int. J. Ophthalmol. 6(3), 390–396 (2013).
- 40 . Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61(2), 158–171 (2009).
- 41 . Design of eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine 6(2), 318–323 (2010).
- 42 . Drug delivery to the eye: special reference to nanoparticles. Int. J. Drug Deliv. 2, 12–21 (2010).
- 43 . Micellar solubilisation of drugs. J. Pharm. Pharm. Sci. 8(2), 147–163 (2005).
- 44 Carbohydrate-based micelle clusters which enhance hydrophobic drug bioavailability by up to 1 order of magnitude. Biomacromolecules 7(12), 3452–3459 (2006).
- 45 . Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 71(3), 445–462 (2009).
- 46 Designing dendrimers for ocular drug delivery. Eur. J. Med. Chem. 45(1), 326–334 (2010).
- 47 Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol. Vis. 12, 415–421 (2006).
- 48 . Cytotoxicity of potential ocular permeation enhancers evaluated on rabbit and human corneal epithelial cell lines. Toxicol. Lett. 122, 1–8 (2001).
- 49 . Gelucire44/14 as a novel absorption enhancer for drugs with different hydrophobicities: in vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 100(8), 3186–3195 (2011). •• Reveals a nonirritating strategy for permeability enhancement of topically applied ocular drug delivery.
- 50 . Gelucire 44/14 based immediate release formulations for poorly water-soluble drugs. Drug Dev. Ind. Pharm. 39(5), 791–798 (2013).
- 51 . Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998).
- 52 . Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS PharmSciTech 4(3), 1–12 (2003).
- 53 . Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 28(4), 353–369 (2002).
- 54 . Ocular drug delivery: an overview. Int. J. Biomed. Adv. Res. 2(5), 167–187 (2011).
- 55 . Hydrogels in mucosal delivery. Ther. Deliv. 3(4), 535–555 (2012).
- 56 . Mucoadhesive drug-delivery systems. J. Pharm. Bioallied Sci. 3(1), 89–100 (2011).
- 57 . Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 11(6), 748–764 (2011).
- 58 . Effect of polaxomer 407 gel on miotic activity of pilocarpine nitrate in rabbits. Int. J. Pharm. 12(2–3), 147–152 (2004).
- 59 . A novel polaxamers/hyaluronic acid in situ forming hydrogel for drug delivery: rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 70(1), 199–206 (2008).
- 60 . In vitro evaluation of pluronic F127 based controlled release ocular delivery systems for pilocarpine. J. Pharm. Sci. 87(2), 226–230 (1998).
- 61 . Chitosan in situ gelation for improved drug loading and retention in polaxamer 407 gels. Int. J. Pharm. 409(1–2), 19–21 (2011).
- 62 . A polaxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 75(2), 186–193 (2010).
- 63 . Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and polaxamer/chitosan in situ forming gel. Eur. J. Pharm. Biopharm. 79(2), 320–327 (2011).
- 64 . Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 120(3), 186–194 (2007).
- 65 . Phase transition of aqueous solutions of poly(N-isopropylmethacrylamide). J. Phys. Chem. 93(8), 3311–3313 (1989).
- 66 . Design and characterization of bioadhesive in-situ gelling ocular inserts of gatifloxacin sesquihydrate. Daru J. Pharm. Sci. 16(1), 1–8 (2008).
- 67 . The development and use of in situ formed gels triggered by pH. In: Biopharmaceutics of Ocular Drug Delivery. Edman P (Ed.). (CRC Press, FL, USA (1993). Ed.). 82–90.
- 68 . PS-60: a new gel-forming polysaccharide. In: Solution Properties of Polysaccharides. Brand DA (Ed.). (ACS Symposium Series, Washington, DC, USA, 111–124 (1981). Ed.).
- 69 . Ion-activated in situ gelling systems for antisense oligodeoxynucleotide delivery to the ocular surface. Mol. Pharm. 8(6), 2282–2290 (2011).
- 70 . Ion-activated, Gelrite®-based in situ ophthalmic gels of pefloxacin mesylate: comparison with conventional eye drops. Drug Deliv. 13(3), 215–219 (2006).
- 71 . Alginate as immobilization matrix for cells. Trends Biotechnol. 8, 71–78 (1990).
- 72 . Mucoadhesive polymers: means of improving the mucoadhesive properties of drug-delivery system. J. Chem. Pharm. Res. 2(5), 418–432 (2010).
- 73 . Mucoadhesive drug-delivery system: an overview. J. Adv. Pharm. Technol. Res. 1(4), 381–387 (2010).
- 74 . The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 57(11), 1556–1568 (2005).
- 75 . Bioadhesive delivery systems for mucosal vaccine delivery. J. Drug Target. 18(10), 752–770 (2010).
- 76 . Critical review on mucoadhesive drug-delivery system. Hygeia J. D. Med. 4(1), 7–28 (2012).
- 77 Hydrogen-bonded complexes and blends of poly(acrylic acid) and methylellulose: nanoparticles and mucoadhesive films for ocular delivery of riboflavin. Macromol. Biosci. 14(2), 225–234 (2014).
- 78 . Biodegradable nanoparticles for drug delivery and targeting. Curr. opin. Solid State Mater. Sci. 6, 319–327 (2002).
- 79 . Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone biotherapy. Mol. Pharm. 7(2), 576–585 (2010).
- 80 . Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 340(1–2), 126–133 (2007).
- 81 . The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with ocular mucosa. Eur. J. Pharm. Sci. 20(1), 73–81 (2003).
- 82 . Thiolated mucoadhesive and PEGylated nonmucoadhesive organosilica nanoparticles from 3-mercaptopropropyltrimethoxysilane. Langmuir 27, 9551–9556 (2011).
- 83 . Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release 136(3), 247–253 (2009).
- 84 Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration. Invest. Ophthalmol. Vis. Sci. 51(6), 3162–3170 (2010).
- 85 . Ocular inserts for topical delivery. Adv. Drug Deliv. Rev. 16(1), 95–106 (1995).
- 86 . Review on ocular inserts. Int. J. Pharm. Tech. Res. 1(2), 164–169 (2009).
- 87 . Recent advances in ocular drug-delivery systems. Polymers 3(1), 193–221 (2011).
- 88 Permeability of human amniotic membrane to ofloxacin in vitro. Invest. Ophthalmol. Vis. Sci. 51(2), 1024–1027 (2010).
- 89 Drug reservoir function of human amniotic membrane. J. Ocul. Pharmacol. Ther. 27(4), 323–326 (2011).
- 90 The concept of ocular inserts as drug-delivery systems: an overview. Asian J. Pharm. 2(4), 192–200 (2008).
- 91 . Effect of chitosan on in vitro release and ocular delivery of ofloxacin from erodible inserts based on poly(ethylene oxide). Int. J. Pharm. 248(1–2), 115–122 (2002).
- 92 . Mucoadhesive ocular insert based on thiolated poly(acrylic acid): development and in vivo evaluation in humans. J. Control. Release 89(3), 419–428 (2003). •• Developed a well-tolerated crosslinked mucoadhesive drug-delivery system based on thiolated poly(acrylic acid), which offered sustained drug release with good resistance to elimination.
- 93 . Effect of physical cross-linking on in vitro and ex vivo permeation of indomethacin from polyvinyl alcohol ocular inserts. Indian J. Pharm. Sci. 65(2), 146–151 (2003).
- 94 . Design and evaluation of polymeric controlled release natamycin ocular inserts. J. Sci. Eng. Technol. 6(1), 108–115 (2010).
- 95 . Cross-linked hydrophilic polymers and articles made therefrom. (1965). Patent: US 3220960
- 96 . Timolol uptake and release by imprinted soft contact lenses made of N, N-diethylacrylamide and methacrylic acid. J. Control. Release 83(2), 223–230 (2006).
- 97 . Molecularly imprinted therapeutic contact lenses. Expert Opin. Drug Deliv. Rev. 7(6), 765–780 (2010).
- 98 . The role of living/controlled radical polymerization in the formation of improved imprinted polymers. J. Mol. Recognit. 25(6), 361–369 (2012).
- 99 . Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 54(1), 149–161 (2002).
- 100 . Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 113(3), 236–244 (2006).
- 101 . Release of vancomycin and gentamicin from contact lens versus a fibrin coating applied to a contact lens. Invest. Ophthalmol. Vis. Sci. 53(4), 1946–1952 (2012).
- 102 A drug-eluting contact lens. Invest. Ophthalmol. Vis. Sci. 50(7), 3346–3352 (2009).
- 103 A prototype antifungal contact lens. Invest. Ophthalmol. Vis. Sci. 52(9), 6286–6291 (2011).
- 104 . Ophthalmic drug delivery through contact lenses. Invest. Ophthalmol. Vis. Sci. 45(7), 2342–2347 (2004).
- 105 . Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int. J. Pharm. 292(1–2), 95–117 (2005).
- 106 . Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 33(7), 2289–2300 (2012).
- 107 . Poly(hydroxyethyl methacrylate-co-methacryalated-β-cyclodextrin) hydrogels: synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomater. 4(3), 745–755 (2008).
- 108 Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 30(7), 1348–1355 (2009).
- 109 . Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 6(2), 486–493 (2010).
- 110 . Recent perspectives in ocular drug delivery. Pharm. Res. 26(5), 1197–1216 (2009).
- 111 Intraocular nanoparticle drug delivery: a pilot study using an aerosol during pars plana vitrectomy. Invest. Ophthalmol. Vis. Sci. 48(11), 5243–5249 (2007).
- 112 Hydrophilic acrylic intraocular lens as a drug-delivery system for fourth-generations fluoroquinolones. J. Cataract Refract. Surg. 32(10), 1717–1721 (2006).
- 113 Rayner C-flex®/Superflex® Monofocal IOL. http://www.rayner.com/products/c-flex-superflex
- 114 An adaptable HPLC method for the analysis of frequently used antibiotics in ocular samples. J. Chromatogr. B 878(26), 2421–2426 (2010).
- 115 STAAR Surgical. http://staar.com/products/collamer-iols/
- 116 Impregnation of an intraocular lens for ophthalmic drug delivery. Curr. Drug Deliv. 5(2), 102–107 (2008).
- 117 Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release. 167(1), 76–84 (2013).
- 118 . Recent patents on ocular drug-delivery systems. Recent Pat. Drug Deliv. Formul. 2(1), 1–8 (2008).
- 119 Vitrasert®. http://www.bioportfolio.com/resources/drug/18877/Vitrasert.html
- 120 Retisert®. http://www.psivida.com/products-retisert.html
- 121 Medidur insert technology. (February 2014). . http://www.retinalphysician.com/printarticle.aspx?articleID=100862
- 122 . Intravitreal steroids in the management of macular oedema. Acta Ophthalmol. Scand. 84(6), 722–733 (2006).
- 123 . The promise of implantable drug-delivery systems. American Academy of Ophthalmology. (June 2014). http://www.aao.org?publications/eyenet/201003/feature.cfm
- 124 Posurdex implant technology. Kuppermann BD. (2007). http://www.retinalphysician.com/printarticle.aspx?articleID=100863
- 125 . Randomised clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology 106(2), 223–231 (1999).
- 126 . An experimental steroid responsive model of ocular inflammation in rabbits using an SLT frequency doubled Q switched Nd:YAG laser. J. Ocul. Pharmacol. Ther. 29(7), 663–669 (2013).
- 127 . Dendrimers as drug carriers:applications in different routes of drug administration. J. Pharm. Sci. 97(1), 123–143 (2008).
- 128 . Dendrimer cross-linked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 27(26), 4608–4617 (2006).
- 129 . Pars plana vitrectomy. (2013). http://emedicine.medscape.com/article/1844160-overview#a01
- 130 . Nanoparticle in-situ gels as vitreous humor substitutes for ocular diseases. (2011). Patent: WO 2011135400A1










